Abstract
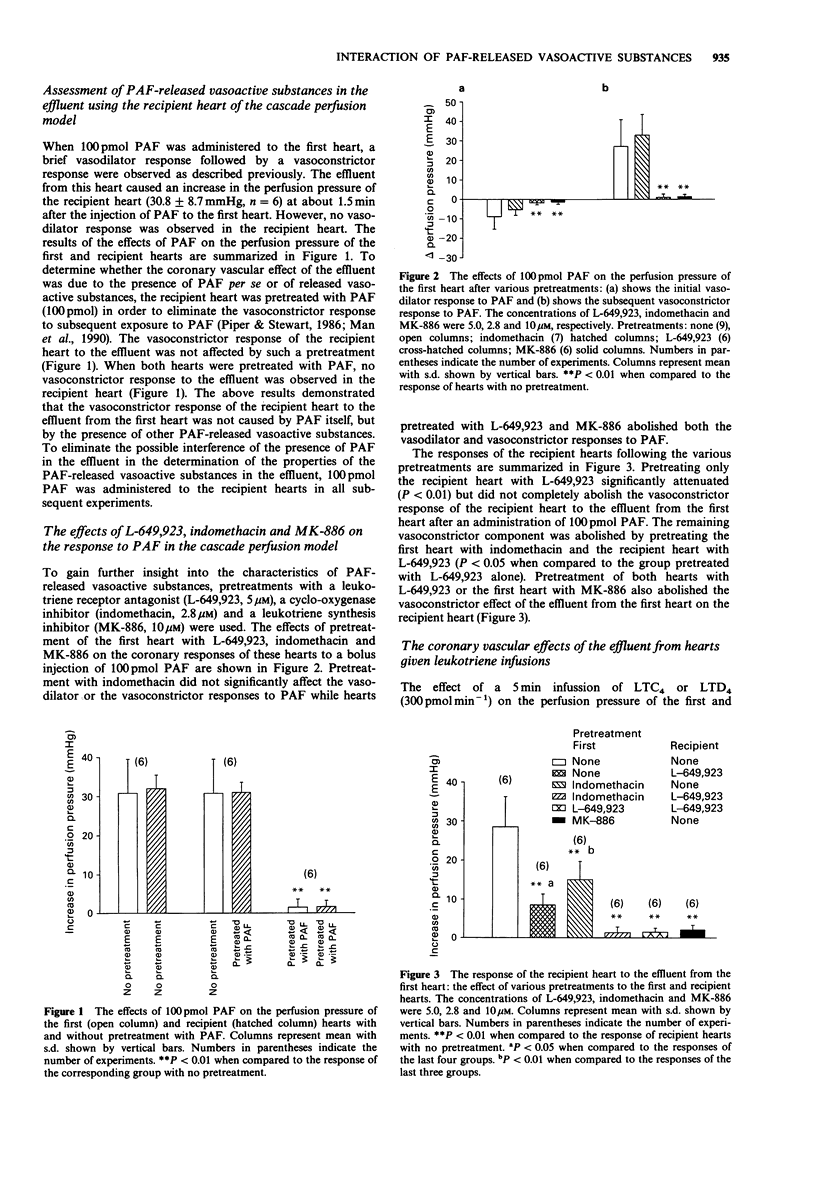
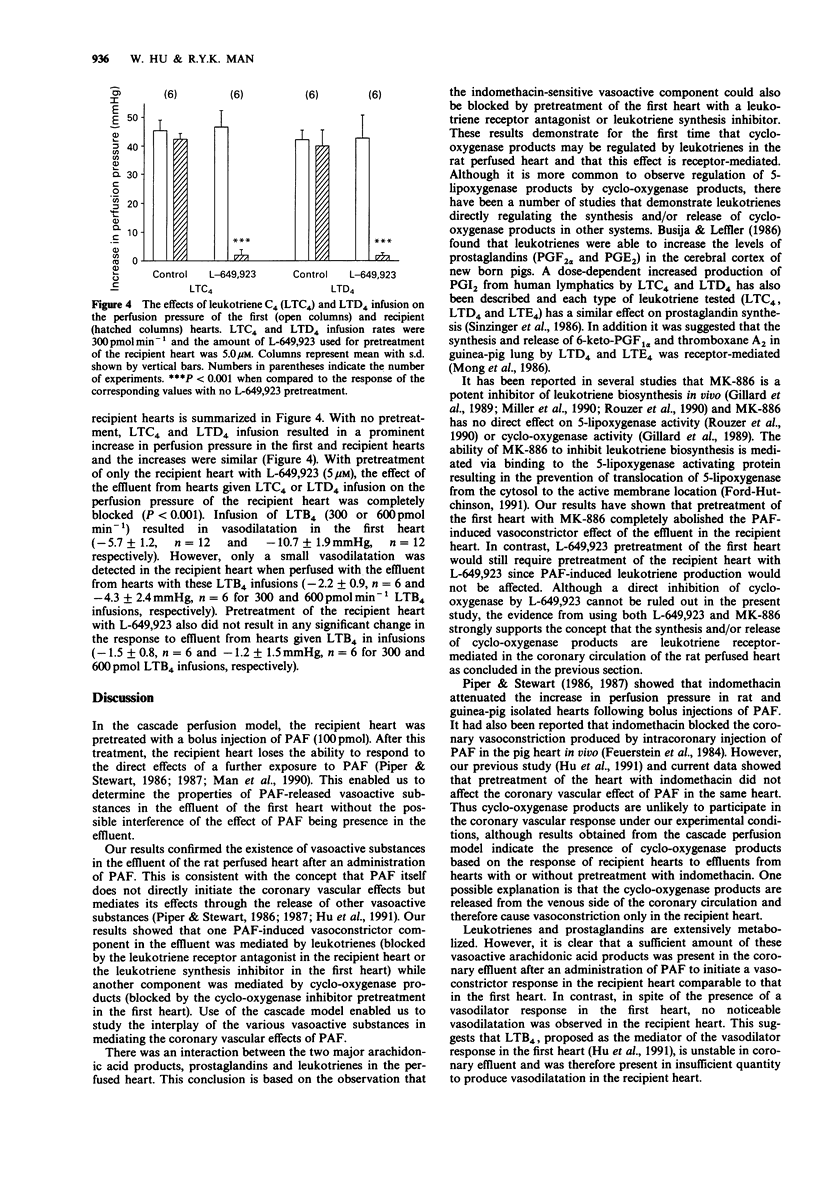
1. The coronary vascular effects of platelet-activating factor (PAF) have been intensively studied and it has been proposed that they are mediated by the release of vasoactive substances. In this study, a cascade perfusion model using two rat perfused hearts was developed to investigate the properties of PAF-released vasoactive substances and the interplay of these substances. The properties of the vasoactive substances after an injection of PAF (100 pmol) in the rat perfused heart were examined by collecting the effluent from the first heart for the perfusion of a second (recipient) heart. The presence of vasoconstrictor substances in the effluent was characterized by an increase in the perfusion pressure of the recipient heart. 2. Previous exposure of the recipient heart of PAF (100 pmol) abolished the response of the heart to subsequent administration of PAF, but did not affect the response of the recipient heart to the effluent. This suggested that the coronary vasoconstrictor response of the recipient heart was not due to the presence of PAF in the effluent but to other vasoactive substances. 3. Pretreatment of the recipient heart with the leukotriene receptor antagonist, L-649,923 (5 microM), partially reduced the vasoconstrictor effect of the effluent. Pretreatment of the first heart with indomethacin (2.8 microM) also partially reduced the vasoconstrictor effect of the effluent. The combination of indomethacin pretreatment of the first heart and L-649,923 pretreatment of the recipient heart completely abolished the vasoconstrictor effect of the effluent suggesting that both prostaglandins and leukotrienes are involved in the vasoconstrictor effect of the effluent. 4. Pretreatment of both hearts with L-649,923 or the first heart with the leukotriene synthesis inhibitor (MK-886, 10 microM) completely abolished the vasoconstrictor effect of the effluent.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Braquet P., Touqui L., Shen T. Y., Vargaftig B. B. Perspectives in platelet-activating factor research. Pharmacol Rev. 1987 Jun;39(2):97–145. [PubMed] [Google Scholar]
- Busija D. W., Leffler C. W. Leukotrienes increase levels of prostanoids in cerebrospinal fluid in piglets. Prostaglandins. 1986 Dec;32(6):803–811. doi: 10.1016/0090-6980(86)90091-2. [DOI] [PubMed] [Google Scholar]
- Ezra D., Laurindo F. R., Czaja J. F., Snyder F., Goldstein R. E., Feuerstein G. Cardiac and coronary consequences of intracoronary platelet activating factor infusion in the domestic pig. Prostaglandins. 1987 Jul;34(1):41–57. doi: 10.1016/0090-6980(87)90261-9. [DOI] [PubMed] [Google Scholar]
- Feuerstein G., Boyd L. M., Ezra D., Goldstein R. E. Effect of platelet-activating factor on coronary circulation of the domestic pig. Am J Physiol. 1984 Mar;246(3 Pt 2):H466–H471. doi: 10.1152/ajpheart.1984.246.3.H466. [DOI] [PubMed] [Google Scholar]
- Fiedler V. B., Mardin M., Abram T. S. Comparison of cardiac and hemodynamic effects of platelet-activating factor-acether and leukotriene D4 in anesthetized dogs. Basic Res Cardiol. 1987 Mar-Apr;82(2):197–208. doi: 10.1007/BF01907067. [DOI] [PubMed] [Google Scholar]
- Ford-Hutchinson A. W. FLAP: a novel drug target for inhibiting the synthesis of leukotrienes. Trends Pharmacol Sci. 1991 Feb;12(2):68–70. doi: 10.1016/0165-6147(91)90500-r. [DOI] [PubMed] [Google Scholar]
- Gillard J., Ford-Hutchinson A. W., Chan C., Charleson S., Denis D., Foster A., Fortin R., Leger S., McFarlane C. S., Morton H. L-663,536 (MK-886) (3-[1-(4-chlorobenzyl)-3-t-butyl-thio-5-isopropylindol-2-yl]-2,2 - dimethylpropanoic acid), a novel, orally active leukotriene biosynthesis inhibitor. Can J Physiol Pharmacol. 1989 May;67(5):456–464. doi: 10.1139/y89-073. [DOI] [PubMed] [Google Scholar]
- Hu W., Kinnaird A. A., Man R. Y. Mechanisms of the coronary vascular effects of platelet-activating factor in the rat perfused heart. Br J Pharmacol. 1991 May;103(1):1097–1102. doi: 10.1111/j.1476-5381.1991.tb12306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson C. V., Schumacher W. A., Kunkel S. L., Driscoll E. M., Lucchesi B. R. Platelet-activating factor and the release of a platelet-derived coronary artery vasodilator substance in the canine. Circ Res. 1986 Feb;58(2):218–229. doi: 10.1161/01.res.58.2.218. [DOI] [PubMed] [Google Scholar]
- Jones T. R., Young R., Champion E., Charette L., Denis D., Ford-Hutchinson A. W., Frenette R., Gauthier J. Y., Guindon Y., Kakushima M. L-649,923, sodium (beta S*, gamma R*)-4-(3-(4-acetyl-3-hydroxy-2-propylphenoxy)-propylthio)- gamma-hydroxy-beta-methylbenzenebutanoate, a selective, orally active leukotriene receptor antagonist. Can J Physiol Pharmacol. 1986 Aug;64(8):1068–1075. doi: 10.1139/y86-183. [DOI] [PubMed] [Google Scholar]
- Levi R., Burke J. A., Guo Z. G., Hattori Y., Hoppens C. M., McManus L. M., Hanahan D. J., Pinckard R. N. Acetyl glyceryl ether phosphorylcholine (AGEPC). A putative mediator of cardiac anaphylaxis in the guinea pig. Circ Res. 1984 Feb;54(2):117–124. doi: 10.1161/01.res.54.2.117. [DOI] [PubMed] [Google Scholar]
- Man R. Y., Hu W., Kinnaird A. A. Coronary vascular response to platelet-activating factor in the perfused rat heart. J Lipid Mediat. 1990 Mar-Apr;2(2):75–83. [PubMed] [Google Scholar]
- Mehta J., Wargovich T., Nichols W. W. Biphasic effects of platelet-activating factor on coronary blood flow in anesthetized dog. Prostaglandins Leukot Med. 1986 Jan;21(1):87–95. doi: 10.1016/0262-1746(86)90166-6. [DOI] [PubMed] [Google Scholar]
- Miller D. K., Gillard J. W., Vickers P. J., Sadowski S., Léveillé C., Mancini J. A., Charleson P., Dixon R. A., Ford-Hutchinson A. W., Fortin R. Identification and isolation of a membrane protein necessary for leukotriene production. Nature. 1990 Jan 18;343(6255):278–281. doi: 10.1038/343278a0. [DOI] [PubMed] [Google Scholar]
- Mong S., Wu H. L., Clark M. A., Gleason J. G., Crooke S. T. Leukotriene D4 receptor-mediated synthesis and release of arachidonic acid metabolites in guinea pig lung: induction of thromboxane and prostacyclin biosynthesis by leukotriene D4. J Pharmacol Exp Ther. 1986 Oct;239(1):63–70. [PubMed] [Google Scholar]
- Montrucchio G., Camussi G., Tetta C., Emanuelli G., Orzan F., Libero L., Brusca A. Intravascular release of platelet-activating factor during atrial pacing. Lancet. 1986 Aug 2;2(8501):293–293. doi: 10.1016/s0140-6736(86)92118-5. [DOI] [PubMed] [Google Scholar]
- Piper P. J., Stewart A. G. Antagonism of vasoconstriction induced by platelet-activating factor in guinea-pig perfused hearts by selective platelet-activating factor receptor antagonists. Br J Pharmacol. 1987 Apr;90(4):771–783. doi: 10.1111/j.1476-5381.1987.tb11231.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper P. J., Stewart A. G. Coronary vasoconstriction in the rat, isolated perfused heart induced by platelet-activating factor is mediated by leukotriene C4. Br J Pharmacol. 1986 Jul;88(3):595–605. doi: 10.1111/j.1476-5381.1986.tb10240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouzer C. A., Ford-Hutchinson A. W., Morton H. E., Gillard J. W. MK886, a potent and specific leukotriene biosynthesis inhibitor blocks and reverses the membrane association of 5-lipoxygenase in ionophore-challenged leukocytes. J Biol Chem. 1990 Jan 25;265(3):1436–1442. [PubMed] [Google Scholar]
- Schweitzer P., Madamba S., Siggins G. R. Arachidonic acid metabolites as mediators of somatostatin-induced increase of neuronal M-current. Nature. 1990 Aug 2;346(6283):464–467. doi: 10.1038/346464a0. [DOI] [PubMed] [Google Scholar]
- Sinzinger H., Kaliman J., Mannheimer E. Effect of leukotrienes C4 and D4 on prostaglandin I2-liberation from human lymphatics. Lymphology. 1986 Jun;19(2):79–81. [PubMed] [Google Scholar]


