Abstract
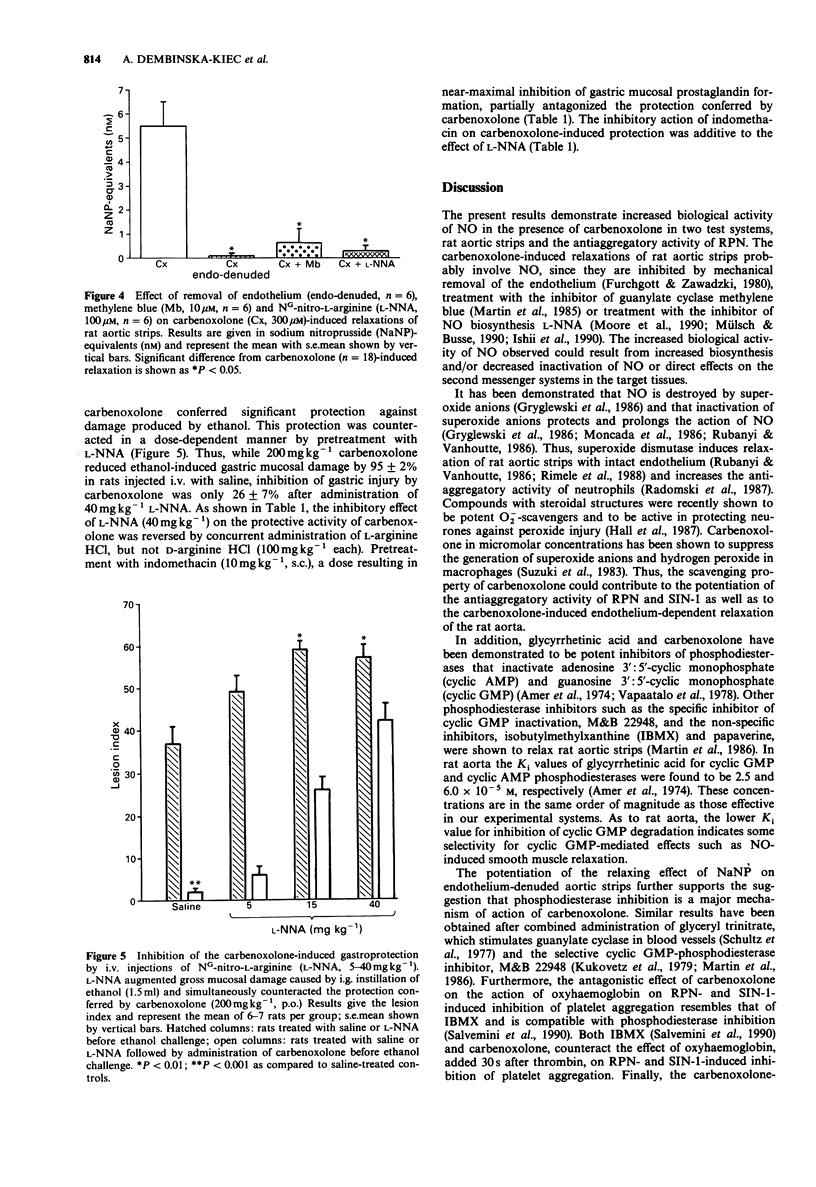
1. The interactions between carbenoxolone and nitric oxide (NO) were examined by investigating their effects on human platelet aggregation, on rat aortic strips precontracted by phenylephrine and on protection of rat gastric mucosa against ethanol-induced injury. 2. Carbenoxolone (100-300 microM) caused a significant and concentration-dependent potentiation of rat peritoneal neutrophil (RPN)- 3-morpholino-syndnonimine (SIN-1)- or iloprost-induced inhibition of platelet aggregation. Higher concentrations (500 microM) of carbenoxolone alone markedly inhibited platelet aggregation. Pretreatment with carbenoxolone (100-300 microM) antagonized the reversal of the RPN- or SIN-1-induced antiaggregatory effect by oxyhaemoglobin (10 microM). 3. Rat aortic strips with intact endothelium precontracted by phenylephrine (0.1-0.3 microM) were relaxed by carbenoxolone (100-300 microM) in a concentration-dependent manner. Relaxations were abolished by mechanical removal of the endothelium or by incubation with methylene blue (10 microM) or NG-nitro-L-arginine (L-NNA, 100 microM). Sodium nitroprusside (10 nM)-induced relaxations of endothelium-denuded rat aortic strips were potentiated by carbenoxolone (100 microM). . The carbenoxolone (200 mg kg-1, p.o.)-induced gastroprotection against ethanol was antagonized by L-NNA (5-40 mg kg-1) in a dose-dependent manner. Pretreatment of rats with indomethacin (10 mg kg-1, s.c.) increased the effect of L-NNA. 5. The results suggest that the activity of carbenoxolone in the experimental systems tested is due to phosphodiesterase inhibition, although radical scavenging properties of the drug could contribute to some of the effects observed. In the rat gastric mucosa both increased prostaglandin levels and effects on the NO system could contribute to the protective action of carbenoxolone.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amer M. S., McKinney G. R., Akcasu A. Effect of glycyrrhetinic acid on the cyclic nucleotide system of the rat stomach. Biochem Pharmacol. 1974 Nov 15;23(22):3085–3092. doi: 10.1016/0006-2952(74)90593-0. [DOI] [PubMed] [Google Scholar]
- Baron J. H. Effect of carbenoxolone sodium on human gastric acid secretion. Gut. 1977 Sep;18(9):721–722. doi: 10.1136/gut.18.9.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derelanko M. J., Long J. F. Carbenoxolone sodium protects rat gastric mucosa against ethanol-induced necrosis. Proc Soc Exp Biol Med. 1981 Mar;166(3):394–397. doi: 10.3181/00379727-166-41080. [DOI] [PubMed] [Google Scholar]
- Furchgott R. F., Zawadzki J. V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980 Nov 27;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Gryglewski R. J., Palmer R. M., Moncada S. Superoxide anion is involved in the breakdown of endothelium-derived vascular relaxing factor. Nature. 1986 Apr 3;320(6061):454–456. doi: 10.1038/320454a0. [DOI] [PubMed] [Google Scholar]
- Hagel J., Renner H., Hirsch M., Weig G., Kaduk B., Ruppin H., Domschke W. Gastric cytoprotection by antacids and papaverine in rats. Hepatogastroenterology. 1982 Dec;29(6):271–274. [PubMed] [Google Scholar]
- Hall E. D., McCall J. M., Chase R. L., Yonkers P. A., Braughler J. M. A nonglucocorticoid steroid analog of methylprednisolone duplicates its high-dose pharmacology in models of central nervous system trauma and neuronal membrane damage. J Pharmacol Exp Ther. 1987 Jul;242(1):137–142. [PubMed] [Google Scholar]
- Holzer P., Pabst M. A., Lippe I. T., Peskar B. M., Peskar B. A., Livingston E. H., Guth P. H. Afferent nerve-mediated protection against deep mucosal damage in the rat stomach. Gastroenterology. 1990 Apr;98(4):838–848. doi: 10.1016/0016-5085(90)90005-l. [DOI] [PubMed] [Google Scholar]
- Ishii K., Chang B., Kerwin J. F., Jr, Huang Z. J., Murad F. N omega-nitro-L-arginine: a potent inhibitor of endothelium-derived relaxing factor formation. Eur J Pharmacol. 1990 Feb 6;176(2):219–223. doi: 10.1016/0014-2999(90)90531-a. [DOI] [PubMed] [Google Scholar]
- Kukovetz W. R., Holzmann S., Wurm A., Pöch G. Evidence for cyclic GMP-mediated relaxant effects of nitro-compounds in coronary smooth muscle. Naunyn Schmiedebergs Arch Pharmacol. 1979 Dec;310(2):129–138. doi: 10.1007/BF00500277. [DOI] [PubMed] [Google Scholar]
- Martin W., Furchgott R. F., Villani G. M., Jothianandan D. Phosphodiesterase inhibitors induce endothelium-dependent relaxation of rat and rabbit aorta by potentiating the effects of spontaneously released endothelium-derived relaxing factor. J Pharmacol Exp Ther. 1986 May;237(2):539–547. [PubMed] [Google Scholar]
- Martin W., Villani G. M., Jothianandan D., Furchgott R. F. Selective blockade of endothelium-dependent and glyceryl trinitrate-induced relaxation by hemoglobin and by methylene blue in the rabbit aorta. J Pharmacol Exp Ther. 1985 Mar;232(3):708–716. [PubMed] [Google Scholar]
- Moncada S., Palmer R. M., Gryglewski R. J. Mechanism of action of some inhibitors of endothelium-derived relaxing factor. Proc Natl Acad Sci U S A. 1986 Dec;83(23):9164–9168. doi: 10.1073/pnas.83.23.9164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore P. K., al-Swayeh O. A., Chong N. W., Evans R. A., Gibson A. L-NG-nitro arginine (L-NOARG), a novel, L-arginine-reversible inhibitor of endothelium-dependent vasodilatation in vitro. Br J Pharmacol. 1990 Feb;99(2):408–412. doi: 10.1111/j.1476-5381.1990.tb14717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mülsch A., Busse R. NG-nitro-L-arginine (N5-[imino(nitroamino)methyl]-L-ornithine) impairs endothelium-dependent dilations by inhibiting cytosolic nitric oxide synthesis from L-arginine. Naunyn Schmiedebergs Arch Pharmacol. 1990 Jan-Feb;341(1-2):143–147. doi: 10.1007/BF00195071. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Stepney R. J., Higgs G. A., Eakins K. E. Chemokinetic activity of arachidonic and lipoxygenase products on leuocyctes of different species. Prostaglandins. 1980 Aug;20(2):411–418. doi: 10.1016/s0090-6980(80)80058-x. [DOI] [PubMed] [Google Scholar]
- Peskar B. M. Effect of carbenoxolone on prostaglandin synthesizing and metabolizing enzymes and correlation with gastric mucosal carbenoxolone concentrations. Scand J Gastroenterol Suppl. 1980;65:109–114. [PubMed] [Google Scholar]
- Peskar B. M., Holland A., Peskar B. A. Effect of carbenoxolone on prostaglandin synthesis and degradation. J Pharm Pharmacol. 1976 Feb;28(2):146–148. doi: 10.1111/j.2042-7158.1976.tb04113.x. [DOI] [PubMed] [Google Scholar]
- Peskar B. M., Lange K., Hoppe U., Peskar B. A. Ethanol stimulates formation of leukotriene C4 in rat gastric mucosa. Prostaglandins. 1986 Feb;31(2):283–293. doi: 10.1016/0090-6980(86)90054-7. [DOI] [PubMed] [Google Scholar]
- Peskar B. M., Respondek M., Müller K. M., Peskar B. A. A role for nitric oxide in capsaicin-induced gastroprotection. Eur J Pharmacol. 1991 May 30;198(1):113–114. doi: 10.1016/0014-2999(91)90572-8. [DOI] [PubMed] [Google Scholar]
- Radomski M. W., Palmer R. M., Moncada S. Comparative pharmacology of endothelium-derived relaxing factor, nitric oxide and prostacyclin in platelets. Br J Pharmacol. 1987 Sep;92(1):181–187. doi: 10.1111/j.1476-5381.1987.tb11310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radomski M., Moncada S. An improved method for washing of human platelets with prostacyclin. Thromb Res. 1983 May 15;30(4):383–389. doi: 10.1016/0049-3848(83)90230-x. [DOI] [PubMed] [Google Scholar]
- Rapoport R. M., Murad F. Agonist-induced endothelium-dependent relaxation in rat thoracic aorta may be mediated through cGMP. Circ Res. 1983 Mar;52(3):352–357. doi: 10.1161/01.res.52.3.352. [DOI] [PubMed] [Google Scholar]
- Rask-Madsen J., Bukhave K., Madsen P. E., Bekker C. Effect of carbenoxolone on gastric prostaglandin E2 levels in patients with peptic ulcer disease following vagal and pentagastrin stimulation. Eur J Clin Invest. 1983 Aug;13(4):351–356. doi: 10.1111/j.1365-2362.1983.tb00112.x. [DOI] [PubMed] [Google Scholar]
- Rimele T. J., Sturm R. J., Adams L. M., Henry D. E., Heaslip R. J., Weichman B. M., Grimes D. Interaction of neutrophils with vascular smooth muscle: identification of a neutrophil-derived relaxing factor. J Pharmacol Exp Ther. 1988 Apr;245(1):102–111. [PubMed] [Google Scholar]
- Rubanyi G. M., Vanhoutte P. M. Superoxide anions and hyperoxia inactivate endothelium-derived relaxing factor. Am J Physiol. 1986 May;250(5 Pt 2):H822–H827. doi: 10.1152/ajpheart.1986.250.5.H822. [DOI] [PubMed] [Google Scholar]
- Salvemini D., Radziszewski W., Korbut R., Vane J. The use of oxyhaemoglobin to explore the events underlying inhibition of platelet aggregation induced by NO or NO-donors. Br J Pharmacol. 1990 Dec;101(4):991–995. doi: 10.1111/j.1476-5381.1990.tb14194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz K., Schultz K., Schultz G. Sodium nitroprusside and other smooth muscle-relaxants increase cyclic GMP levels in rat ductus deferens. Nature. 1977 Feb 24;265(5596):750–751. doi: 10.1038/265750a0. [DOI] [PubMed] [Google Scholar]
- Vapaatalo H., Lindén I. B., Metsä-Ketelä T., Kangasaho M., Laustiola K. Effect of carbenoxolone on phosphodiesterase and prostaglandin synthetase activities. Experientia. 1978 Mar 15;34(3):384–385. doi: 10.1007/BF01923050. [DOI] [PubMed] [Google Scholar]
- Wan B. Y., Gottfried S. Cytoprotective action of carbenoxolone sodium on ethanol-induced gastric lesions in rats and its inhibition by indomethacin. J Pharm Pharmacol. 1985 Oct;37(10):739–741. doi: 10.1111/j.2042-7158.1985.tb04956.x. [DOI] [PubMed] [Google Scholar]


