Abstract
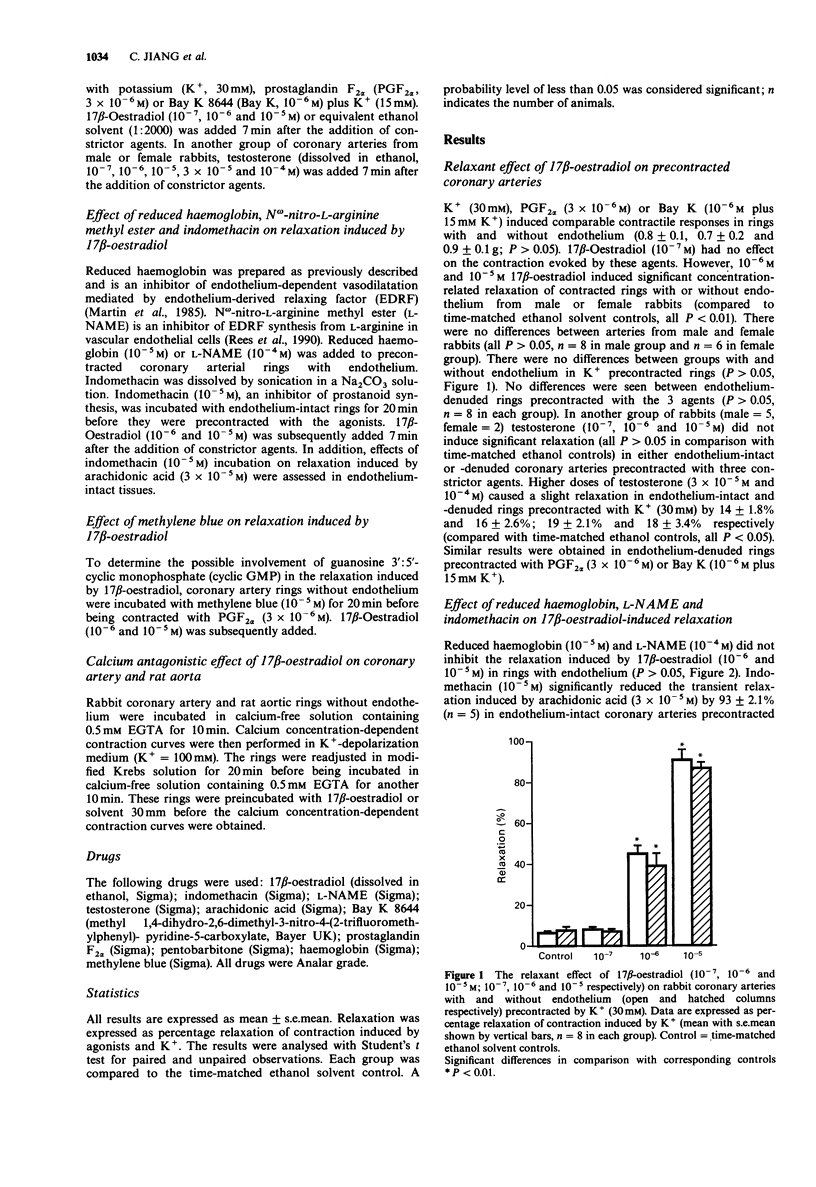
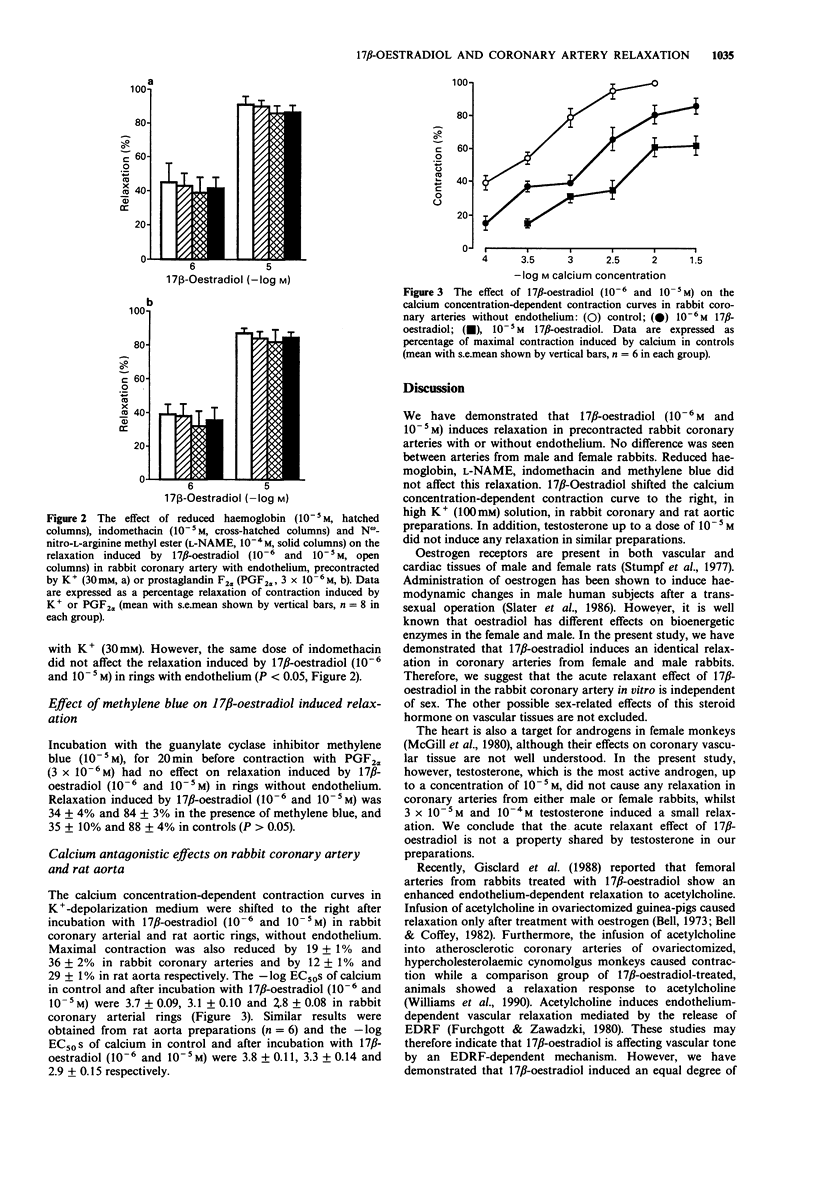
1. We assessed the relaxant effect of 17 beta-oestradiol (10(-7), 10(-6) and 10(-5) M) on rabbit isolated coronary arteries precontracted with prostaglandin F2 alpha (3 x 10(-6) M), high extracellular potassium (30 mM) and Bay K 8644 (10(-6) M) plus high extracellular potassium (15 mM) by measuring isometric tension. 17 beta-Oestradiol (10(-6) and 10(-5) M) induced significant relaxation in coronary arteries from male and female rabbits. No differences were seen between arteries with or without endothelium. There were also no differences between coronary arteries isolated from male and female rabbits. 2. Inhibitors of endothelium-derived relaxing factor and vasodilator prostanoids, namely, reduced haemoglobin, N omega-nitro-L-arginine methyl ester and indomethacin, did not affect the relaxation induced by 17 beta-oestradiol in endothelium-intact coronary arteries. 3. Methylene blue, an inhibitor of guanylate cyclase, did not affect the coronary artery relaxation induced by 17 beta-oestradiol. 4. The calcium concentration-dependent contraction curve in potassium-depolarization medium was shifted to the right by 17 beta-oestradiol (10(-6) and 10(-5) M) in the rabbit coronary artery and rat aorta. The -log EC50s of calcium in control and after incubation with 17 beta-oestradiol (10(-6) and 10(-5) M) were 3.7 +/- 0.09, 3.1 +/- 0.10 and 2.8 +/- 0.08 respectively in rabbit coronary arteries and 3.8 +/- 0.11, 3.3 +/- 0.14 and 2.9 +/- 0.15 in rat aorta. 5. The results indicate that 17 beta-oestradiol induces rabbit coronary artery relaxation by an endothelium-independent mechanism in vitro.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bell C., Coffey C. Factors influencing oestrogen-induced sensitization to acetylcholine of guinea-pig uterine artery. J Reprod Fertil. 1982 Sep;66(1):133–137. doi: 10.1530/jrf.0.0660133. [DOI] [PubMed] [Google Scholar]
- Bell C. Oestrogen-induced sensitization of the uterine artery of the guinea-pig to acetylcholine. Br J Pharmacol. 1973 Dec;49(4):595–601. doi: 10.1111/j.1476-5381.1973.tb08535.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. M., Kunze D. L., Yatani A. The agonist effect of dihydropyridines on Ca channels. Nature. 1984 Oct 11;311(5986):570–572. doi: 10.1038/311570a0. [DOI] [PubMed] [Google Scholar]
- Bush T. L., Barrett-Connor E., Cowan L. D., Criqui M. H., Wallace R. B., Suchindran C. M., Tyroler H. A., Rifkind B. M. Cardiovascular mortality and noncontraceptive use of estrogen in women: results from the Lipid Research Clinics Program Follow-up Study. Circulation. 1987 Jun;75(6):1102–1109. doi: 10.1161/01.cir.75.6.1102. [DOI] [PubMed] [Google Scholar]
- Campbell S., Whitehead M. Oestrogen therapy and the menopausal syndrome. Clin Obstet Gynaecol. 1977 Apr;4(1):31–47. [PubMed] [Google Scholar]
- Chang W. C., Nakao J., Orimo H., Murota S. Stimulation of prostacyclin biosynthetic activity by estradiol in rat aortic smooth muscle cells in culture. Biochim Biophys Acta. 1980 Jul 14;619(1):107–118. [PubMed] [Google Scholar]
- Colditz G. A., Willett W. C., Stampfer M. J., Rosner B., Speizer F. E., Hennekens C. H. Menopause and the risk of coronary heart disease in women. N Engl J Med. 1987 Apr 30;316(18):1105–1110. doi: 10.1056/NEJM198704303161801. [DOI] [PubMed] [Google Scholar]
- De Mey J. G., Claeys M., Vanhoutte P. M. Endothelium-dependent inhibitory effects of acetylcholine, adenosine triphosphate, thrombin and arachidonic acid in the canine femoral artery. J Pharmacol Exp Ther. 1982 Jul;222(1):166–173. [PubMed] [Google Scholar]
- Downing S. J., Hollingsworth M., Miller M. The influence of oestrogen and progesterone on the actions of two calcium entry blockers in the rat uterus. J Endocrinol. 1988 Aug;118(2):251–258. doi: 10.1677/joe.0.1180251. [DOI] [PubMed] [Google Scholar]
- Ettinger B., Genant H. K., Cann C. E. Long-term estrogen replacement therapy prevents bone loss and fractures. Ann Intern Med. 1985 Mar;102(3):319–324. doi: 10.7326/0003-4819-102-3-319. [DOI] [PubMed] [Google Scholar]
- Ferreira S. H., Moncada S., Vane J. R. Further experiments to establish that the analgesic action of aspirin-like drugs depends on the inhibition of prostaglandin biosynthesis. Br J Pharmacol. 1973 Mar;47(3):629P–630P. [PMC free article] [PubMed] [Google Scholar]
- Ferreira S. H., Moncada S., Vane J. R. Indomethacin and aspirin abolish prostaglandin release from the spleen. Nat New Biol. 1971 Jun 23;231(25):237–239. doi: 10.1038/newbio231237a0. [DOI] [PubMed] [Google Scholar]
- Ford S. P., Reynolds L. P., Farley D. B., Bhatnagar R. K., Van Orden D. E. Interaction of ovarian steroids and periarterial alpha 1-adrenergic receptors in altering uterine blood flow during the estrous cycle of gilts. Am J Obstet Gynecol. 1984 Nov 1;150(5 Pt 1):480–484. doi: 10.1016/s0002-9378(84)90424-1. [DOI] [PubMed] [Google Scholar]
- Furchgott R. F., Zawadzki J. V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980 Nov 27;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Gisclard V., Miller V. M., Vanhoutte P. M. Effect of 17 beta-estradiol on endothelium-dependent responses in the rabbit. J Pharmacol Exp Ther. 1988 Jan;244(1):19–22. [PubMed] [Google Scholar]
- Gisclard V., Miller V. M., Vanhoutte P. M. Effect of 17 beta-estradiol on endothelium-dependent responses in the rabbit. J Pharmacol Exp Ther. 1988 Jan;244(1):19–22. [PubMed] [Google Scholar]
- Godsland I. F., Wynn V., Crook D., Miller N. E. Sex, plasma lipoproteins, and atherosclerosis: prevailing assumptions and outstanding questions. Am Heart J. 1987 Dec;114(6):1467–1503. doi: 10.1016/0002-8703(87)90552-7. [DOI] [PubMed] [Google Scholar]
- Gruchow H. W., Anderson A. J., Barboriak J. J., Sobocinski K. A. Postmenopausal use of estrogen and occlusion of coronary arteries. Am Heart J. 1988 May;115(5):954–963. doi: 10.1016/0002-8703(88)90063-4. [DOI] [PubMed] [Google Scholar]
- Henry P. D., Bentley K. I. Suppression of atherogenesis in cholesterol-fed rabbit treated with nifedipine. J Clin Invest. 1981 Nov;68(5):1366–1369. doi: 10.1172/JCI110384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannel W. B., Hjortland M. C., McNamara P. M., Gordon T. Menopause and risk of cardiovascular disease: the Framingham study. Ann Intern Med. 1976 Oct;85(4):447–452. doi: 10.7326/0003-4819-85-4-447. [DOI] [PubMed] [Google Scholar]
- Lichtlen P. R., Hugenholtz P. G., Rafflenbeul W., Hecker H., Jost S., Deckers J. W. Retardation of angiographic progression of coronary artery disease by nifedipine. Results of the International Nifedipine Trial on Antiatherosclerotic Therapy (INTACT). INTACT Group Investigators. Lancet. 1990 May 12;335(8698):1109–1113. doi: 10.1016/0140-6736(90)91121-p. [DOI] [PubMed] [Google Scholar]
- Magness R. R., Rosenfeld C. R. Local and systemic estradiol-17 beta: effects on uterine and systemic vasodilation. Am J Physiol. 1989 Apr;256(4 Pt 1):E536–E542. doi: 10.1152/ajpendo.1989.256.4.E536. [DOI] [PubMed] [Google Scholar]
- Martin W., Villani G. M., Jothianandan D., Furchgott R. F. Selective blockade of endothelium-dependent and glyceryl trinitrate-induced relaxation by hemoglobin and by methylene blue in the rabbit aorta. J Pharmacol Exp Ther. 1985 Mar;232(3):708–716. [PubMed] [Google Scholar]
- Matthews K. A., Meilahn E., Kuller L. H., Kelsey S. F., Caggiula A. W., Wing R. R. Menopause and risk factors for coronary heart disease. N Engl J Med. 1989 Sep 7;321(10):641–646. doi: 10.1056/NEJM198909073211004. [DOI] [PubMed] [Google Scholar]
- McCalden T. A. The inhibitory action of oestradiol-17-beta and progesterone on venous smooth muscle. Br J Pharmacol. 1975 Feb;53(2):183–192. doi: 10.1111/j.1476-5381.1975.tb07348.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGill H. C., Jr, Anselmo V. C., Buchanan J. M., Sheridan P. J. The heart is a target organ for androgen. Science. 1980 Feb 15;207(4432):775–777. doi: 10.1126/science.6766222. [DOI] [PubMed] [Google Scholar]
- Raddino R., Manca C., Poli E., Bolognesi R., Visioli O. Effects of 17 beta-estradiol on the isolated rabbit heart. Arch Int Pharmacodyn Ther. 1986 May;281(1):57–65. [PubMed] [Google Scholar]
- Rees D. D., Palmer R. M., Schulz R., Hodson H. F., Moncada S. Characterization of three inhibitors of endothelial nitric oxide synthase in vitro and in vivo. Br J Pharmacol. 1990 Nov;101(3):746–752. doi: 10.1111/j.1476-5381.1990.tb14151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandahl B., Andersson K. E., Aronsen K. F., Ulmsten U. Effect of the calcium antagonist nifedipine on uterine blood flow in non-treated and oestradiol-treated rats. Gynecol Obstet Invest. 1978;9(5):238–243. doi: 10.1159/000300990. [DOI] [PubMed] [Google Scholar]
- Sarrel P. M. Ovarian hormones and the circulation. Maturitas. 1990 Sep;12(3):287–298. doi: 10.1016/0378-5122(90)90008-t. [DOI] [PubMed] [Google Scholar]
- Slater A. J., Gude N., Clarke I. J., Walters W. A. Haemodynamic changes and left ventricular performance during high-dose oestrogen administration to male transsexuals. Br J Obstet Gynaecol. 1986 Jun;93(6):532–538. doi: 10.1111/j.1471-0528.1986.tb07949.x. [DOI] [PubMed] [Google Scholar]
- Steinleitner A., Stanczyk F. Z., Levin J. H., d'Ablaing G., 3rd, Vijod M. A., Shahbazian V. L., Lobo R. A. Decreased in vitro production of 6-keto-prostaglandin F1 alpha by uterine arteries from postmenopausal women. Am J Obstet Gynecol. 1989 Dec;161(6 Pt 1):1677–1681. doi: 10.1016/0002-9378(89)90949-6. [DOI] [PubMed] [Google Scholar]
- Stice S. L., Ford S. P., Rosazza J. P., Van Orden D. E. Interaction of 4-hydroxylated estradiol and potential-sensitive Ca2+ channels in altering uterine blood flow during the estrous cycle and early pregnancy in gilts. Biol Reprod. 1987 Mar;36(2):369–375. doi: 10.1095/biolreprod36.2.369. [DOI] [PubMed] [Google Scholar]
- Stice S. L., Ford S. P., Rosazza J. P., Van Orden D. E. Role of 4-hydroxylated estradiol in reducing Ca2+ uptake of uterine arterial smooth muscle cells through potential-sensitive channels. Biol Reprod. 1987 Mar;36(2):361–368. doi: 10.1095/biolreprod36.2.361. [DOI] [PubMed] [Google Scholar]
- Stumpf W. E., Sar M., Aumüller G. The heart: a target organ for estradiol. Science. 1977 Apr 15;196(4287):319–321. doi: 10.1126/science.847474. [DOI] [PubMed] [Google Scholar]
- Sullivan J. M., Vander Zwaag R., Lemp G. F., Hughes J. P., Maddock V., Kroetz F. W., Ramanathan K. B., Mirvis D. M. Postmenopausal estrogen use and coronary atherosclerosis. Ann Intern Med. 1988 Mar;108(3):358–363. doi: 10.7326/0003-4819-108-3-358. [DOI] [PubMed] [Google Scholar]
- Takayama T. [Effects of highly concentrated estrogen and progesterone on the contractile mechanism of the uterine smooth muscles]. Nihon Heikatsukin Gakkai Zasshi. 1986 Feb;22(1):43–51. doi: 10.1540/jsmr1965.22.43. [DOI] [PubMed] [Google Scholar]
- Waters D., Lespérance J., Francetich M., Causey D., Théroux P., Chiang Y. K., Hudon G., Lemarbre L., Reitman M., Joyal M. A controlled clinical trial to assess the effect of a calcium channel blocker on the progression of coronary atherosclerosis. Circulation. 1990 Dec;82(6):1940–1953. doi: 10.1161/01.cir.82.6.1940. [DOI] [PubMed] [Google Scholar]
- Williams J. K., Adams M. R., Klopfenstein H. S. Estrogen modulates responses of atherosclerotic coronary arteries. Circulation. 1990 May;81(5):1680–1687. doi: 10.1161/01.cir.81.5.1680. [DOI] [PubMed] [Google Scholar]



