Abstract
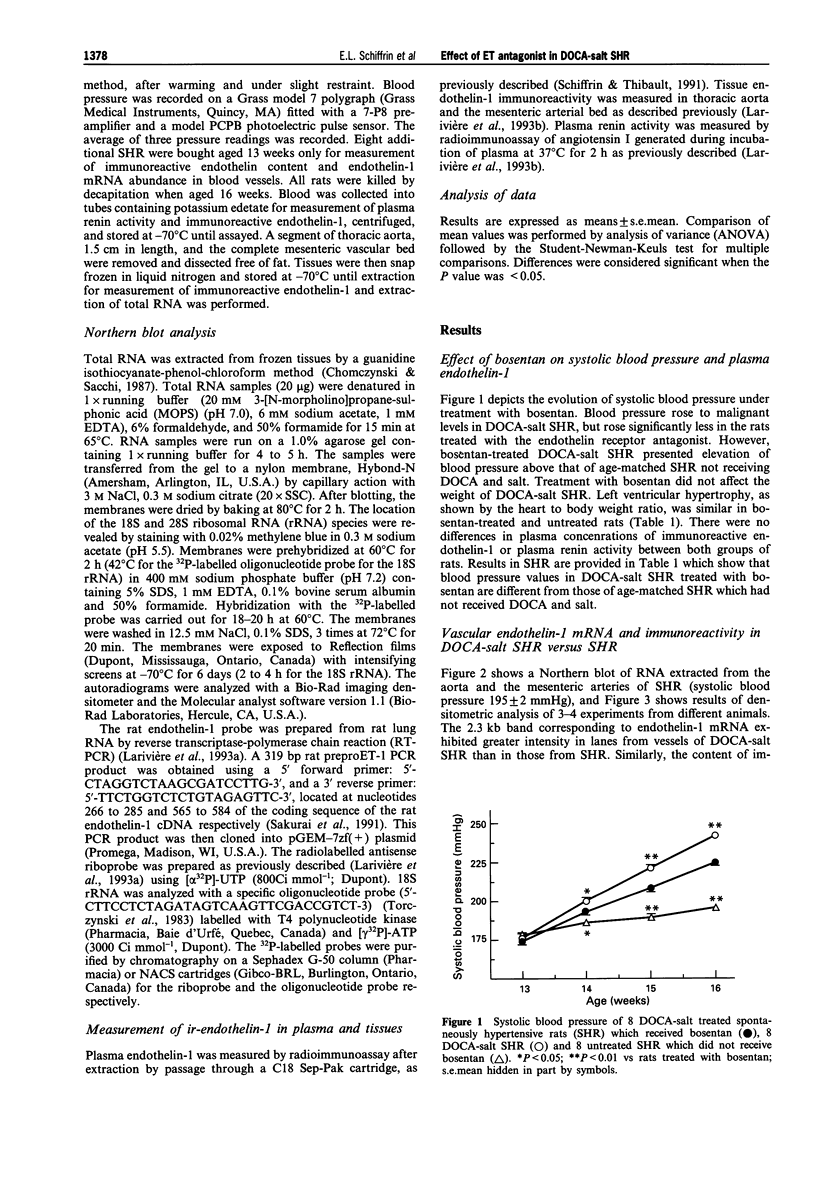
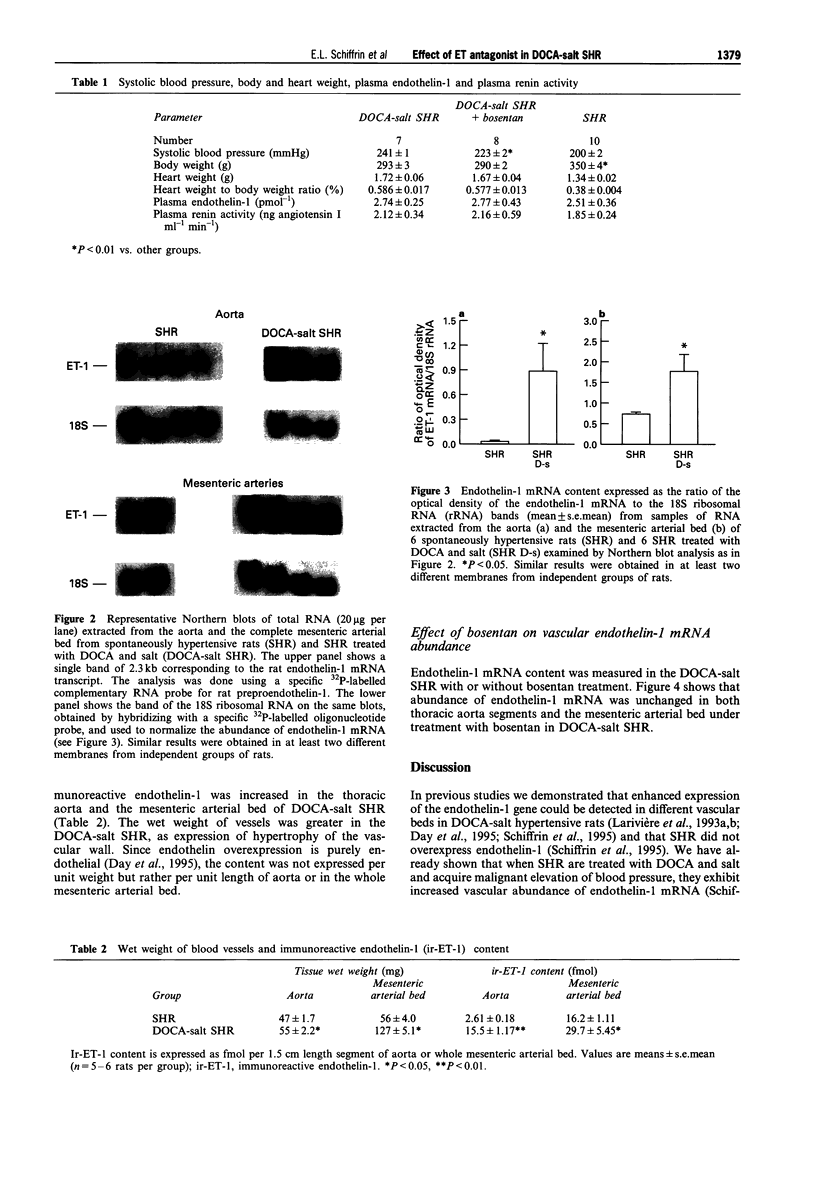
1. Endothelin-1 gene expression is enhanced in aorta and mesenteric arteries, and possibly other vessels, of deoxycorticosterone acetate (DOCA)-salt hypertensive rats but is normal or reduced in spontaneously hypertensive rats (SHR). Bosentan, a mixed ETA/ETB endothelin receptor antagonist, blunts the development of elevated blood pressure of DOCA-salt hypertensive rats but not in SHR. In this study we investigated whether treatment of DOCA-salt SHR with bosentan would result in blunted rise in blood pressure. 2. SHR, aged 13 weeks, were implanted with silastic containing DOCA and offered 1% saline to drink. Systolic blood pressure was measured by the tail-cuff method. Endothelin-1 mRNA abundance in aorta and mesenteric arteries was measured by Northern blot analysis. Content of immunoreactive endothelin in blood vessels was measured by radioimmunoassay. 3. Systolic blood pressure rose less in bosentan-treated DOCA-salt SHR (to 223 +/- 2 mmHg) in comparison to the untreated rats (241 +/- 1), a small but significant difference (P < 0.001). However, blood pressure of bosentan-treated DOCA-salt SHR was still higher than in age-matched SHR. Endothelin-1 mRNA abundance and content of immunoreactive endothelin were increased in the aorta and the mesenteric arterial bed of DOCA-salt SHR, and were unaffected by treatment with bosentan. 4. These data support the hypothesis of a role of endothelin-1 in blood pressure elevation in this hypertensive model with malignant hypertension. They also support the hypothesis that an antihypertensive effect of the mixed ETA/ETB endothelin receptor antagonist, bosentan, is found when experimental hypertensive animals exhibit enhanced endothelin-1 gene expression in blood vessels.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Clozel M., Breu V., Gray G. A., Kalina B., Löffler B. M., Burri K., Cassal J. M., Hirth G., Müller M., Neidhart W. Pharmacological characterization of bosentan, a new potent orally active nonpeptide endothelin receptor antagonist. J Pharmacol Exp Ther. 1994 Jul;270(1):228–235. [PubMed] [Google Scholar]
- Day R., Larivière R., Schiffrin E. L. In situ hybridization shows increased endothelin-1 mRNA levels in endothelial cells of blood vessels of deoxycorticosterone acetate-salt hypertensive rats. Am J Hypertens. 1995 Mar;8(3):294–300. doi: 10.1016/0895-7061(95)96213-4. [DOI] [PubMed] [Google Scholar]
- Imai T., Hirata Y., Emori T., Yanagisawa M., Masaki T., Marumo F. Induction of endothelin-1 gene by angiotensin and vasopressin in endothelial cells. Hypertension. 1992 Jun;19(6 Pt 2):753–757. doi: 10.1161/01.hyp.19.6.753. [DOI] [PubMed] [Google Scholar]
- Inoue A., Yanagisawa M., Kimura S., Kasuya Y., Miyauchi T., Goto K., Masaki T. The human endothelin family: three structurally and pharmacologically distinct isopeptides predicted by three separate genes. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2863–2867. doi: 10.1073/pnas.86.8.2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurihara H., Yoshizumi M., Sugiyama T., Takaku F., Yanagisawa M., Masaki T., Hamaoki M., Kato H., Yazaki Y. Transforming growth factor-beta stimulates the expression of endothelin mRNA by vascular endothelial cells. Biochem Biophys Res Commun. 1989 Mar 31;159(3):1435–1440. doi: 10.1016/0006-291x(89)92270-5. [DOI] [PubMed] [Google Scholar]
- Larivière R., Day R., Schiffrin E. L. Increased expression of endothelin-1 gene in blood vessels of deoxycorticosterone acetate-salt hypertensive rats. Hypertension. 1993 Jun;21(6 Pt 2):916–920. doi: 10.1161/01.hyp.21.6.916. [DOI] [PubMed] [Google Scholar]
- Larivière R., Sventek P., Thibault G., Schiffrin E. L. Endothelin-1 expression in blood vessels of DOCA-salt hypertensive rats treated with the combined ETA/ETB endothelin receptor antagonist bosentan. Can J Physiol Pharmacol. 1995 Mar;73(3):390–398. doi: 10.1139/y95-050. [DOI] [PubMed] [Google Scholar]
- Larivière R., Thibault G., Schiffrin E. L. Increased endothelin-1 content in blood vessels of deoxycorticosterone acetate-salt hypertensive but not in spontaneously hypertensive rats. Hypertension. 1993 Mar;21(3):294–300. doi: 10.1161/01.hyp.21.3.294. [DOI] [PubMed] [Google Scholar]
- Li J. S., Larivière R., Schiffrin E. L. Effect of a nonselective endothelin antagonist on vascular remodeling in deoxycorticosterone acetate-salt hypertensive rats. Evidence for a role of endothelin in vascular hypertrophy. Hypertension. 1994 Aug;24(2):183–188. doi: 10.1161/01.hyp.24.2.183. [DOI] [PubMed] [Google Scholar]
- Li J. S., Schiffrin E. L. Effect of chronic treatment of adult spontaneously hypertensive rats with an endothelin receptor antagonist. Hypertension. 1995 Apr;25(4 Pt 1):495–500. doi: 10.1161/01.hyp.25.4.495. [DOI] [PubMed] [Google Scholar]
- Löffler B. M., Breu V., Clozel M. Effect of different endothelin receptor antagonists and of the novel non-peptide antagonist Ro 46-2005 on endothelin levels in rat plasma. FEBS Lett. 1993 Oct 25;333(1-2):108–110. doi: 10.1016/0014-5793(93)80384-7. [DOI] [PubMed] [Google Scholar]
- Ormsbee H. S., 3rd, Ryan C. F. Production of hypertension with desoxycorticorticosterone acetate-impregnated silicone rubber implants. J Pharm Sci. 1973 Feb;62(2):255–257. doi: 10.1002/jps.2600620215. [DOI] [PubMed] [Google Scholar]
- Sakurai T., Yanagisawa M., Inoue A., Ryan U. S., Kimura S., Mitsui Y., Goto K., Masaki T. cDNA cloning, sequence analysis and tissue distribution of rat preproendothelin-1 mRNA. Biochem Biophys Res Commun. 1991 Feb 28;175(1):44–47. doi: 10.1016/s0006-291x(05)81197-0. [DOI] [PubMed] [Google Scholar]
- Sarzani R., Brecher P., Chobanian A. V. Growth factor expression in aorta of normotensive and hypertensive rats. J Clin Invest. 1989 Apr;83(4):1404–1408. doi: 10.1172/JCI114029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffrin E. L., Deng L. Y. Comparison of effects of angiotensin I-converting enzyme inhibition and beta-blockade for 2 years on function of small arteries from hypertensive patients. Hypertension. 1995 Apr;25(4 Pt 2):699–703. doi: 10.1161/01.hyp.25.4.699. [DOI] [PubMed] [Google Scholar]
- Schiffrin E. L., Thibault G. Plasma endothelin in human essential hypertension. Am J Hypertens. 1991 Apr;4(4 Pt 1):303–308. doi: 10.1093/ajh/4.4.303. [DOI] [PubMed] [Google Scholar]
- Sesoko S., Pegram B. L., Willis G. W., Frohlich E. D. DOCA-salt induced malignant hypertension in spontaneously hypertensive rats. J Hypertens. 1984 Feb;2(1):49–54. doi: 10.1097/00004872-198402000-00009. [DOI] [PubMed] [Google Scholar]
- Stacy D. L., Scott J. W., Granger J. P. Control of renal function during intrarenal infusion of endothelin. Am J Physiol. 1990 May;258(5 Pt 2):F1232–F1236. doi: 10.1152/ajprenal.1990.258.5.F1232. [DOI] [PubMed] [Google Scholar]
- Torczynski R., Bollon A. P., Fuke M. The complete nucleotide sequence of the rat 18S ribosomal RNA gene and comparison with the respective yeast and frog genes. Nucleic Acids Res. 1983 Jul 25;11(14):4879–4890. doi: 10.1093/nar/11.14.4879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagisawa M., Kurihara H., Kimura S., Tomobe Y., Kobayashi M., Mitsui Y., Yazaki Y., Goto K., Masaki T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988 Mar 31;332(6163):411–415. doi: 10.1038/332411a0. [DOI] [PubMed] [Google Scholar]
- Yoshizumi M., Kurihara H., Sugiyama T., Takaku F., Yanagisawa M., Masaki T., Yazaki Y. Hemodynamic shear stress stimulates endothelin production by cultured endothelial cells. Biochem Biophys Res Commun. 1989 Jun 15;161(2):859–864. doi: 10.1016/0006-291x(89)92679-x. [DOI] [PubMed] [Google Scholar]



