Abstract
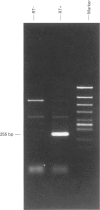
1. In the present study, we determined whether ETA receptors are present on endothelial cells in situ, by use of front-surface fluorometry of fura-2-loaded porcine aortic valvular strips and reverse transcription polymerase chain reaction (RT-PCR). 2. Although endothelin-1 (ET-1) and endothelin-3 (ET-3) induced maximum elevations of cytosolic Ca2+ concentrations ([Ca2+]i) at 10(-7) M, the peak elevations of [Ca2+]i induced by ET-1 were much greater than those induced by ET-3. 3. The application of ET-1 after ET-3 induced an additional increase in [Ca2+]i, while the application of ET-3 after ET-1 had no effect. A selective ETA receptor antagonist, BQ-123, partially inhibited the ET-1-induced Ca2+ transient but had no effect on ET-3-induced Ca2+ transients. These experiments indicated the presence of functioning ETA receptors in addition to ETB receptors in endothelial cells in situ. 4. The sequence of pig lung ETA receptor complimentary DNA (cDNA) was determined by PCR. RT-PCR, using specific primers for pig ETA receptor sequence and total RNA from endothelial cells on the aortic side of the aortic valve, gave the expected size of band. This PCR product was sequenced and was found to be identical to the sequence of the pig lung ETA receptor. 5. The partial sequence of the pig lung ETB receptor was also determined. RT-PCR for the pig ETB receptor revealed that endothelial cells of the aortic valve express ETB receptor messenger RNA (mRNA).(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adachi M., Yang Y. Y., Furuichi Y., Miyamoto C. Cloning and characterization of cDNA encoding human A-type endothelin receptor. Biochem Biophys Res Commun. 1991 Nov 14;180(3):1265–1272. doi: 10.1016/s0006-291x(05)81332-4. [DOI] [PubMed] [Google Scholar]
- Amano K., Hori M., Ozaki H., Matsuda Y., Karaki H. Ca++ mobilization mediated by endothelin ETA receptor in endothelium of rabbit aortic valve. J Pharmacol Exp Ther. 1994 Dec;271(3):1359–1364. [PubMed] [Google Scholar]
- Aoki H., Kobayashi S., Nishimura J., Kanaide H. Sensitivity of G-protein involved in endothelin-1-induced Ca2+ influx to pertussis toxin in porcine endothelial cells in situ. Br J Pharmacol. 1994 Apr;111(4):989–996. doi: 10.1111/j.1476-5381.1994.tb14841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki H., Kobayashi S., Nishimura J., Yamamoto H., Kanaide H. Endothelin induces the Ca(2+)-transient in endothelial cells in situ. Biochem Biophys Res Commun. 1991 Dec 31;181(3):1352–1357. doi: 10.1016/0006-291x(91)92087-z. [DOI] [PubMed] [Google Scholar]
- Arai H., Hori S., Aramori I., Ohkubo H., Nakanishi S. Cloning and expression of a cDNA encoding an endothelin receptor. Nature. 1990 Dec 20;348(6303):730–732. doi: 10.1038/348730a0. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- DeLean A., Munson P. J., Rodbard D. Simultaneous analysis of families of sigmoidal curves: application to bioassay, radioligand assay, and physiological dose-response curves. Am J Physiol. 1978 Aug;235(2):E97–102. doi: 10.1152/ajpendo.1978.235.2.E97. [DOI] [PubMed] [Google Scholar]
- Di Virgilio F., Steinberg T. H., Silverstein S. C. Organic-anion transport inhibitors to facilitate measurement of cytosolic free Ca2+ with fura-2. Methods Cell Biol. 1989;31:453–462. doi: 10.1016/s0091-679x(08)61622-2. [DOI] [PubMed] [Google Scholar]
- Emori T., Hirata Y., Marumo F. Specific receptors for endothelin-3 in cultured bovine endothelial cells and its cellular mechanism of action. FEBS Lett. 1990 Apr 24;263(2):261–264. doi: 10.1016/0014-5793(90)81388-5. [DOI] [PubMed] [Google Scholar]
- Fujitani Y., Oda K., Takimoto M., Inui T., Okada T., Urade Y. Autocrine receptors for endothelins in the primary culture of endothelial cells of human umbilical vein. FEBS Lett. 1992 Feb 17;298(1):79–83. doi: 10.1016/0014-5793(92)80026-d. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hirano K., Kanaide H., Abe S., Nakamura M. Effects of diltiazem on calcium concentrations in the cytosol and on force of contractions in porcine coronary arterial strips. Br J Pharmacol. 1990 Oct;101(2):273–280. doi: 10.1111/j.1476-5381.1990.tb12700.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosoda K., Nakao K., Hiroshi-Arai, Suga S., Ogawa Y., Mukoyama M., Shirakami G., Saito Y., Nakanishi S., Imura H. Cloning and expression of human endothelin-1 receptor cDNA. FEBS Lett. 1991 Aug 5;287(1-2):23–26. doi: 10.1016/0014-5793(91)80007-p. [DOI] [PubMed] [Google Scholar]
- Ihara M., Noguchi K., Saeki T., Fukuroda T., Tsuchida S., Kimura S., Fukami T., Ishikawa K., Nishikibe M., Yano M. Biological profiles of highly potent novel endothelin antagonists selective for the ETA receptor. Life Sci. 1992;50(4):247–255. doi: 10.1016/0024-3205(92)90331-i. [DOI] [PubMed] [Google Scholar]
- Inoue A., Yanagisawa M., Kimura S., Kasuya Y., Miyauchi T., Goto K., Masaki T. The human endothelin family: three structurally and pharmacologically distinct isopeptides predicted by three separate genes. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2863–2867. doi: 10.1073/pnas.86.8.2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe E. A. Physiologic functions of normal endothelial cells. Ann N Y Acad Sci. 1985;454:279–291. doi: 10.1111/j.1749-6632.1985.tb11868.x. [DOI] [PubMed] [Google Scholar]
- Karne S., Jayawickreme C. K., Lerner M. R. Cloning and characterization of an endothelin-3 specific receptor (ETC receptor) from Xenopus laevis dermal melanophores. J Biol Chem. 1993 Sep 5;268(25):19126–19133. [PubMed] [Google Scholar]
- Ku D. D., Nelson J. M., Caulfield J. B., Winn M. J. Release of endothelium-derived relaxing factors from canine cardiac valves. J Cardiovasc Pharmacol. 1990 Aug;16(2):212–218. doi: 10.1097/00005344-199008000-00006. [DOI] [PubMed] [Google Scholar]
- Kuroiwa M., Aoki H., Kobayashi S., Nishimura J., Kanaide H. Role of GTP-protein and endothelium in contraction induced by ethanol in pig coronary artery. J Physiol. 1993 Oct;470:521–537. doi: 10.1113/jphysiol.1993.sp019873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskey R. E., Adams D. J., van Breemen C. Cytosolic [Ca2+] measurements in endothelium of rabbit cardiac valves using imaging fluorescence microscopy. Am J Physiol. 1994 May;266(5 Pt 2):H2130–H2135. doi: 10.1152/ajpheart.1994.266.5.H2130. [DOI] [PubMed] [Google Scholar]
- Li L., van Breemen C. Na(+)-Ca2+ exchange in intact endothelium of rabbit cardiac valve. Circ Res. 1995 Mar;76(3):396–404. doi: 10.1161/01.res.76.3.396. [DOI] [PubMed] [Google Scholar]
- Lin H. Y., Kaji E. H., Winkel G. K., Ives H. E., Lodish H. F. Cloning and functional expression of a vascular smooth muscle endothelin 1 receptor. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3185–3189. doi: 10.1073/pnas.88.8.3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masaki T., Kimura S., Yanagisawa M., Goto K. Molecular and cellular mechanism of endothelin regulation. Implications for vascular function. Circulation. 1991 Oct;84(4):1457–1468. doi: 10.1161/01.cir.84.4.1457. [DOI] [PubMed] [Google Scholar]
- Nakamuta M., Takayanagi R., Sakai Y., Sakamoto S., Hagiwara H., Mizuno T., Saito Y., Hirose S., Yamamoto M., Nawata H. Cloning and sequence analysis of a cDNA encoding human non-selective type of endothelin receptor. Biochem Biophys Res Commun. 1991 May 31;177(1):34–39. doi: 10.1016/0006-291x(91)91944-8. [DOI] [PubMed] [Google Scholar]
- Nishimura J., Kobayashi S., Shikasho T., Kanaide H. Platelet derived growth factor induces c-fos and c-myc mRNA in rat aortic smooth muscle cells in primary culture without elevation of intracellular Ca2+ concentration. Biochem Biophys Res Commun. 1992 Nov 16;188(3):1198–1204. doi: 10.1016/0006-291x(92)91358-w. [DOI] [PubMed] [Google Scholar]
- Ogawa Y., Nakao K., Arai H., Nakagawa O., Hosoda K., Suga S., Nakanishi S., Imura H. Molecular cloning of a non-isopeptide-selective human endothelin receptor. Biochem Biophys Res Commun. 1991 Jul 15;178(1):248–255. doi: 10.1016/0006-291x(91)91806-n. [DOI] [PubMed] [Google Scholar]
- Remuzzi G., Benigni A. Endothelins in the control of cardiovascular and renal function. Lancet. 1993 Sep 4;342(8871):589–593. doi: 10.1016/0140-6736(93)91414-h. [DOI] [PubMed] [Google Scholar]
- Saito Y., Mizuno T., Itakura M., Suzuki Y., Ito T., Hagiwara H., Hirose S. Primary structure of bovine endothelin ETB receptor and identification of signal peptidase and metal proteinase cleavage sites. J Biol Chem. 1991 Dec 5;266(34):23433–23437. [PubMed] [Google Scholar]
- Sakurai T., Yanagisawa M., Takuwa Y., Miyazaki H., Kimura S., Goto K., Masaki T. Cloning of a cDNA encoding a non-isopeptide-selective subtype of the endothelin receptor. Nature. 1990 Dec 20;348(6303):732–735. doi: 10.1038/348732a0. [DOI] [PubMed] [Google Scholar]
- Suzuki S., Kajikuri J., Suzuki A., Itoh T. Effects of endothelin-1 on endothelial cells in the porcine coronary artery. Circ Res. 1991 Nov;69(5):1361–1368. doi: 10.1161/01.res.69.5.1361. [DOI] [PubMed] [Google Scholar]
- Takayanagi R., Kitazumi K., Takasaki C., Ohnaka K., Aimoto S., Tasaka K., Ohashi M., Nawata H. Presence of non-selective type of endothelin receptor on vascular endothelium and its linkage to vasodilation. FEBS Lett. 1991 Apr 22;282(1):103–106. doi: 10.1016/0014-5793(91)80454-b. [DOI] [PubMed] [Google Scholar]
- Ushio-Fukai M., Nishimura J., Aoki H., Kobayashi S., Kanaide H. Endothelin-1 inhibits and enhances contraction of porcine coronary arterial strips with an intact endothelium. Biochem Biophys Res Commun. 1992 Apr 15;184(1):518–524. doi: 10.1016/0006-291x(92)91225-f. [DOI] [PubMed] [Google Scholar]
- Vane J. Endothelins come home to roost. Nature. 1990 Dec 20;348(6303):673–673. doi: 10.1038/348673a0. [DOI] [PubMed] [Google Scholar]
- Vigne P., Ladoux A., Frelin C. Endothelins activate Na+/H+ exchange in brain capillary endothelial cells via a high affinity endothelin-3 receptor that is not coupled to phospholipase C. J Biol Chem. 1991 Mar 25;266(9):5925–5928. [PubMed] [Google Scholar]
- Vigne P., Marsault R., Breittmayer J. P., Frelin C. Endothelin stimulates phosphatidylinositol hydrolysis and DNA synthesis in brain capillary endothelial cells. Biochem J. 1990 Mar 1;266(2):415–420. doi: 10.1042/bj2660415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner T. D., Schmidt H. H., Murad F. Interactions of endothelins and EDRF in bovine native endothelial cells: selective effects of endothelin-3. Am J Physiol. 1992 May;262(5 Pt 2):H1600–H1605. doi: 10.1152/ajpheart.1992.262.5.H1600. [DOI] [PubMed] [Google Scholar]
- Warner T. D., de Nucci G., Vane J. R. Rat endothelin is a vasodilator in the isolated perfused mesentery of the rat. Eur J Pharmacol. 1989 Jan 17;159(3):325–326. doi: 10.1016/0014-2999(89)90167-2. [DOI] [PubMed] [Google Scholar]
- Watanabe H., Miyazaki H., Kondoh M., Masuda Y., Kimura S., Yanagisawa M., Masaki T., Murakami K. Two distinct types of endothelin receptors are present on chick cardiac membranes. Biochem Biophys Res Commun. 1989 Jun 30;161(3):1252–1259. doi: 10.1016/0006-291x(89)91377-6. [DOI] [PubMed] [Google Scholar]
- Yanagisawa M., Kurihara H., Kimura S., Tomobe Y., Kobayashi M., Mitsui Y., Yazaki Y., Goto K., Masaki T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988 Mar 31;332(6163):411–415. doi: 10.1038/332411a0. [DOI] [PubMed] [Google Scholar]