Abstract
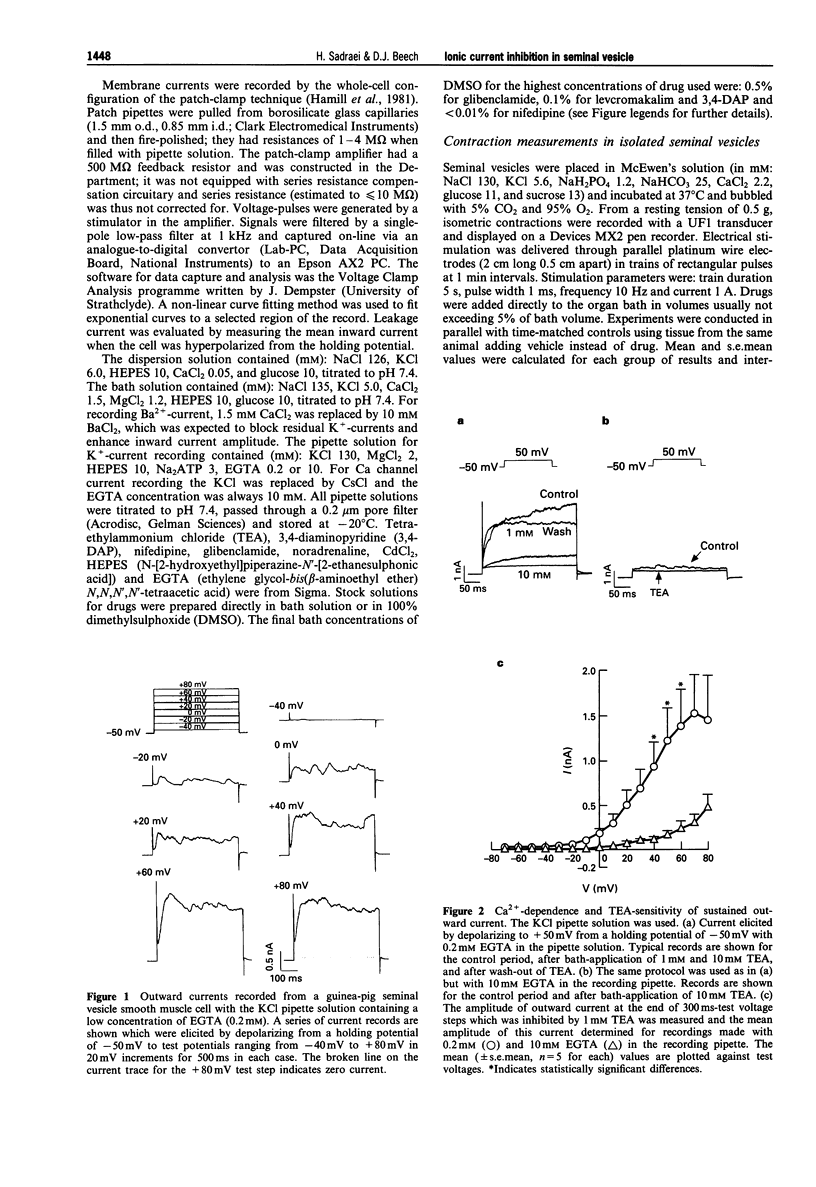
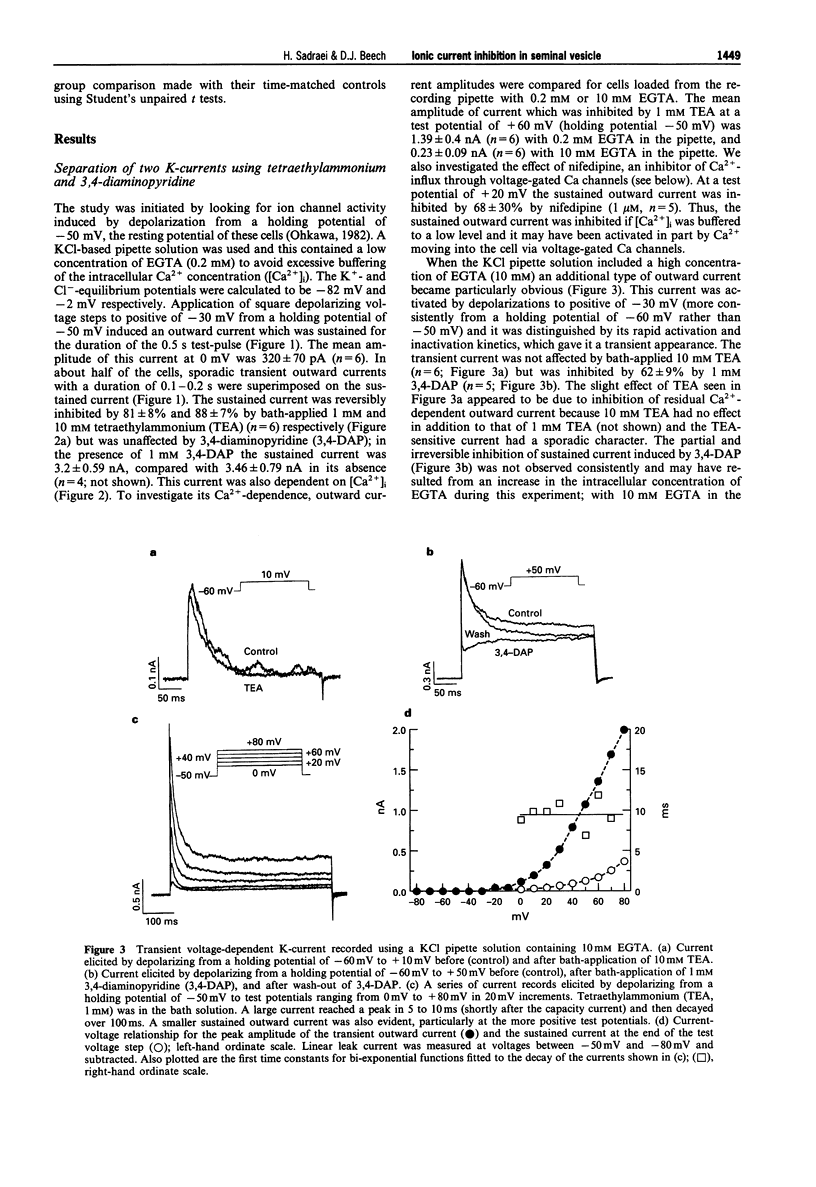
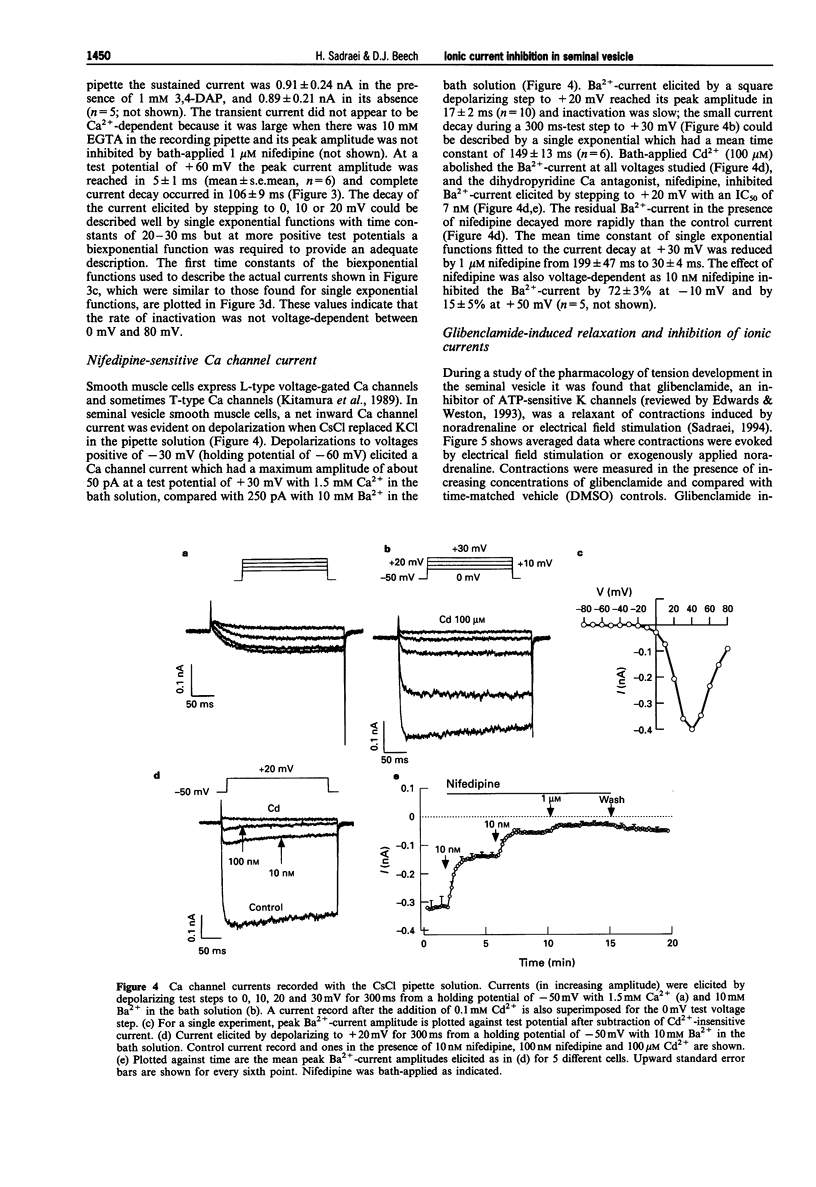
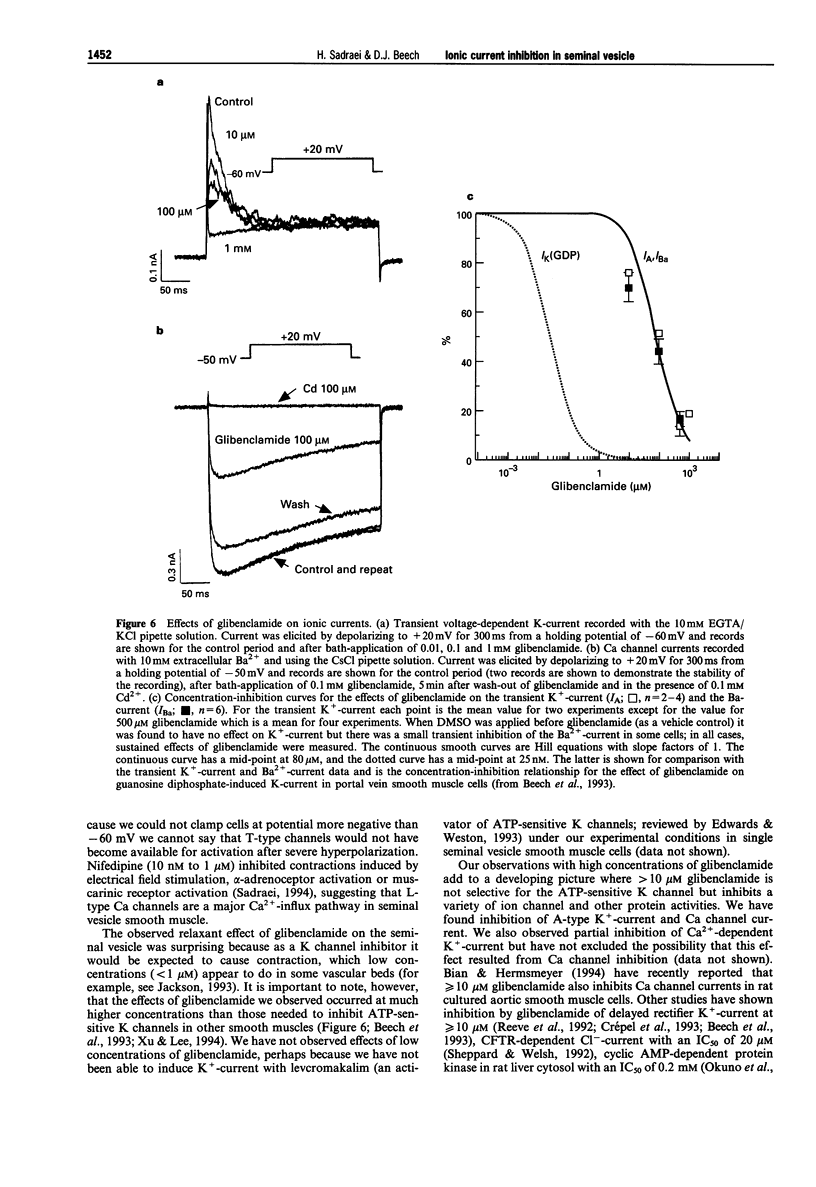
1. Whole-cell voltage-clamp recordings were made from smooth muscle cells isolated from guinea-pig seminal vesicle. 2. When the recording pipette solution contained 130 mM KCl and a low concentration of EGTA (0.2 mM), a dominant outward current was elicited by depolarization to positive of -30 mV from a holding potential of -50 mV. The current was non-inactivating, stimulated by intracellular Ca2+ and blocked by bath-applied 1 mM tetraethylammonium but not 1 mM 3,4 diaminopyridine. 3. If 10 mM EGTA was added to the KCl pipette solution and the holding potential was -50 mV, or more negative, the major current elicited by depolarization to positive of -30 mV was an A-type K(+)-current. This current inactivated rapidly (within 100 ms) and was blocked by bath-applied 1 mM 3,4-diaminopyridine but not 10 mM tetraethylammonium. 4. An inward voltage-gated Ca channel current was observed on depolarization to positive of -30 mV with 1.5 mM Ca2+ or 10 mM Ba2+ in the bath solution and when Ca+ replaced K+ in the pipette. The Ba(2+)-current was shown to be abolished by bath-applied 100 microM Cd2+ and inhibited by 90% by 1 microM nifedipine, and thus appeared to be carried by L-type Ca channels. 5. High concentrations of glibenclamide (10-500 microM) inhibited A-type K(+)-current, Ba(2+)-current and contraction of the whole tissue induced by noradrenaline or electrical field stimulation.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF
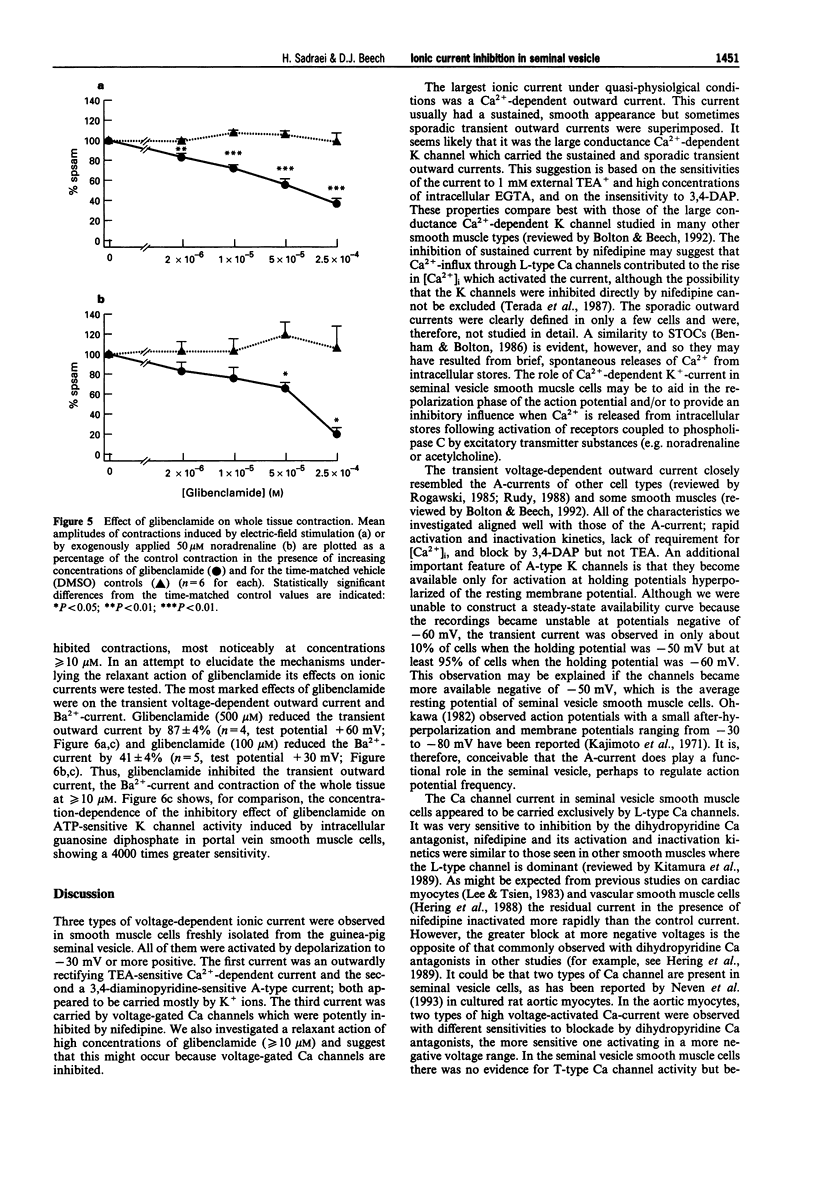


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Al-Zuhair A., Gosling J. A., Dixon J. S. Observations on the structure and autonomic innervation of the guinea-pig seminal vesicle and ductus deferens. J Anat. 1975 Sep;120(Pt 1):81–93. [PMC free article] [PubMed] [Google Scholar]
- Beech D. J., Bolton T. B. Two components of potassium current activated by depolarization of single smooth muscle cells from the rabbit portal vein. J Physiol. 1989 Nov;418:293–309. doi: 10.1113/jphysiol.1989.sp017841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beech D. J., Zhang H., Nakao K., Bolton T. B. Single channel and whole-cell K-currents evoked by levcromakalim in smooth muscle cells from the rabbit portal vein. Br J Pharmacol. 1993 Oct;110(2):583–590. doi: 10.1111/j.1476-5381.1993.tb13850.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benham C. D., Bolton T. B. Spontaneous transient outward currents in single visceral and vascular smooth muscle cells of the rabbit. J Physiol. 1986 Dec;381:385–406. doi: 10.1113/jphysiol.1986.sp016333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bian K., Hermsmeyer K. Glyburide actions on the dihydropyridine-sensitive Ca2+ channel in rat vascular muscle. J Vasc Res. 1994 Sep-Oct;31(5):256–264. doi: 10.1159/000159051. [DOI] [PubMed] [Google Scholar]
- Chopra L. C., Twort C. H., Ward J. P. Direct action of BRL 38227 and glibenclamide on intracellular calcium stores in cultured airway smooth muscle of rabbit. Br J Pharmacol. 1992 Feb;105(2):259–260. doi: 10.1111/j.1476-5381.1992.tb14242.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clavert A., Cranz C., Bollack C. Functions of the seminal vesicle. Andrologia. 1990;22 (Suppl 1):185–192. doi: 10.1111/j.1439-0272.1990.tb02084.x. [DOI] [PubMed] [Google Scholar]
- Crépel V., Krnjević K., Ben-Ari Y. Sulphonylureas reduce the slowly inactivating D-type outward current in rat hippocampal neurons. J Physiol. 1993 Jul;466:39–54. [PMC free article] [PubMed] [Google Scholar]
- Edwards G., Weston A. H. The pharmacology of ATP-sensitive potassium channels. Annu Rev Pharmacol Toxicol. 1993;33:597–637. doi: 10.1146/annurev.pa.33.040193.003121. [DOI] [PubMed] [Google Scholar]
- Gordienko D. V., Clausen C., Goligorsky M. S. Ionic currents and endothelin signaling in smooth muscle cells from rat renal resistance arteries. Am J Physiol. 1994 Feb;266(2 Pt 2):F325–F341. doi: 10.1152/ajprenal.1994.266.2.F325. [DOI] [PubMed] [Google Scholar]
- Grissmer S., Nguyen A. N., Aiyar J., Hanson D. C., Mather R. J., Gutman G. A., Karmilowicz M. J., Auperin D. D., Chandy K. G. Pharmacological characterization of five cloned voltage-gated K+ channels, types Kv1.1, 1.2, 1.3, 1.5, and 3.1, stably expressed in mammalian cell lines. Mol Pharmacol. 1994 Jun;45(6):1227–1234. [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hering S., Beech D. J., Bolton T. B., Lim S. P. Action of nifedipine or BAY K8644 is dependent on calcium channel state in single smooth muscle cells from rabbit ear artery. Pflugers Arch. 1988 May;411(5):590–592. doi: 10.1007/BF00582383. [DOI] [PubMed] [Google Scholar]
- Hering S., Kleppisch T., Timin E. N., Bodewei R. Characterization of the calcium channel state transitions induced by the enantiomers of the 1,4-dihydropyridine Sandoz 202 791 in neonatal rat heart cells. A nonmodulated receptor model. Pflugers Arch. 1989 Sep;414(6):690–700. doi: 10.1007/BF00582137. [DOI] [PubMed] [Google Scholar]
- Hisada T., Kurachi Y., Sugimoto T. Properties of membrane currents in isolated smooth muscle cells from guinea-pig trachea. Pflugers Arch. 1990 Apr;416(1-2):151–161. doi: 10.1007/BF00370237. [DOI] [PubMed] [Google Scholar]
- Holevinsky K. O., Fan Z., Frame M., Makielski J. C., Groppi V., Nelson D. J. ATP-sensitive K+ channel opener acts as a potent Cl- channel inhibitor in vascular smooth muscle cells. J Membr Biol. 1994 Jan;137(1):59–70. doi: 10.1007/BF00234998. [DOI] [PubMed] [Google Scholar]
- Jackson W. F. Arteriolar tone is determined by activity of ATP-sensitive potassium channels. Am J Physiol. 1993 Nov;265(5 Pt 2):H1797–H1803. doi: 10.1152/ajpheart.1993.265.5.H1797. [DOI] [PubMed] [Google Scholar]
- Kajimoto N., Kirpekar S. M., Wakade A. R. An investigation of spontaneous potentials recorded from the smooth-muscle cells of the guinea-pig seminal vesicle. J Physiol. 1972 Jul;224(1):105–119. doi: 10.1113/jphysiol.1972.sp009883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura K., Inoue Y., Inoue R., Ohya Y., Terada K., Okabe K., Kuriyama H. Properties of the inward ionic currents and their regulating agents in smooth muscle cells. Gen Physiol Biophys. 1989 Aug;8(4):289–312. [PubMed] [Google Scholar]
- Lang R. J. Identification of the major membrane currents in freshly dispersed single smooth muscle cells of guinea-pig ureter. J Physiol. 1989 May;412:375–395. doi: 10.1113/jphysiol.1989.sp017622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. S., Tsien R. W. Mechanism of calcium channel blockade by verapamil, D600, diltiazem and nitrendipine in single dialysed heart cells. Nature. 1983 Apr 28;302(5911):790–794. doi: 10.1038/302790a0. [DOI] [PubMed] [Google Scholar]
- Neveu D., Nargeot J., Richard S. Two high-voltage-activated, dihydropyridine-sensitive Ca2+ channel currents with distinct electrophysiological and pharmacological properties in cultured rat aortic myocytes. Pflugers Arch. 1993 Jun;424(1):45–53. doi: 10.1007/BF00375101. [DOI] [PubMed] [Google Scholar]
- Ohkawa H. Excitatory junction potentials recorded from the circular smooth muscles of the guinea-pig seminal vesicle. Tohoku J Exp Med. 1982 Jan;136(1):89–102. doi: 10.1620/tjem.136.89. [DOI] [PubMed] [Google Scholar]
- Ohya Y., Terada K., Kitamura K., Kuriyama H. Membrane currents recorded from a fragment of rabbit intestinal smooth muscle cell. Am J Physiol. 1986 Sep;251(3 Pt 1):C335–C346. doi: 10.1152/ajpcell.1986.251.3.C335. [DOI] [PubMed] [Google Scholar]
- Okabe K., Kajioka S., Nakao K., Kitamura K., Kuriyama H., Weston A. H. Actions of cromakalim on ionic currents recorded from single smooth muscle cells of the rat portal vein. J Pharmacol Exp Ther. 1990 Feb;252(2):832–839. [PubMed] [Google Scholar]
- Okuno S., Inaba M., Nishizawa Y., Inoue A., Morii H. Effect of tolbutamide and glyburide on cAMP-dependent protein kinase activity in rat liver cytosol. Diabetes. 1988 Jul;37(7):857–861. doi: 10.2337/diab.37.7.857. [DOI] [PubMed] [Google Scholar]
- Reeve H. L., Vaughan P. F., Peers C. Glibenclamide inhibits a voltage-gated K+ current in the human neuroblastoma cell line SH-SY5Y. Neurosci Lett. 1992 Jan 20;135(1):37–40. doi: 10.1016/0304-3940(92)90130-y. [DOI] [PubMed] [Google Scholar]
- Rudy B. Diversity and ubiquity of K channels. Neuroscience. 1988 Jun;25(3):729–749. doi: 10.1016/0306-4522(88)90033-4. [DOI] [PubMed] [Google Scholar]
- Sheppard D. N., Welsh M. J. Effect of ATP-sensitive K+ channel regulators on cystic fibrosis transmembrane conductance regulator chloride currents. J Gen Physiol. 1992 Oct;100(4):573–591. doi: 10.1085/jgp.100.4.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terada K., Kitamura K., Kuriyama H. Different inhibitions of the voltage-dependent K+ current by Ca2+ antagonists in the smooth muscle cell membrane of rabbit small intestine. Pflugers Arch. 1987 May;408(6):558–564. doi: 10.1007/BF00581156. [DOI] [PubMed] [Google Scholar]
- Xu X., Lee K. S. Characterization of the ATP-inhibited K+ current in canine coronary smooth muscle cells. Pflugers Arch. 1994 May;427(1-2):110–120. doi: 10.1007/BF00585949. [DOI] [PubMed] [Google Scholar]


