Abstract
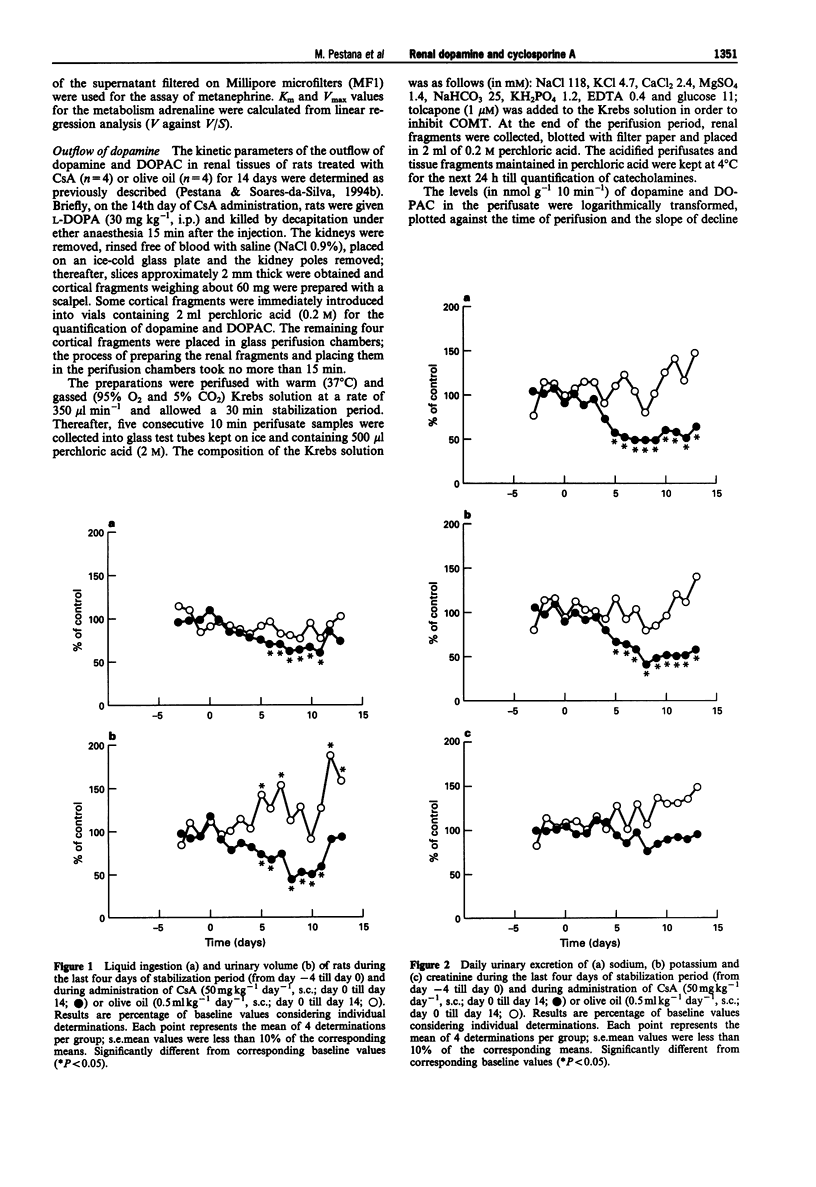
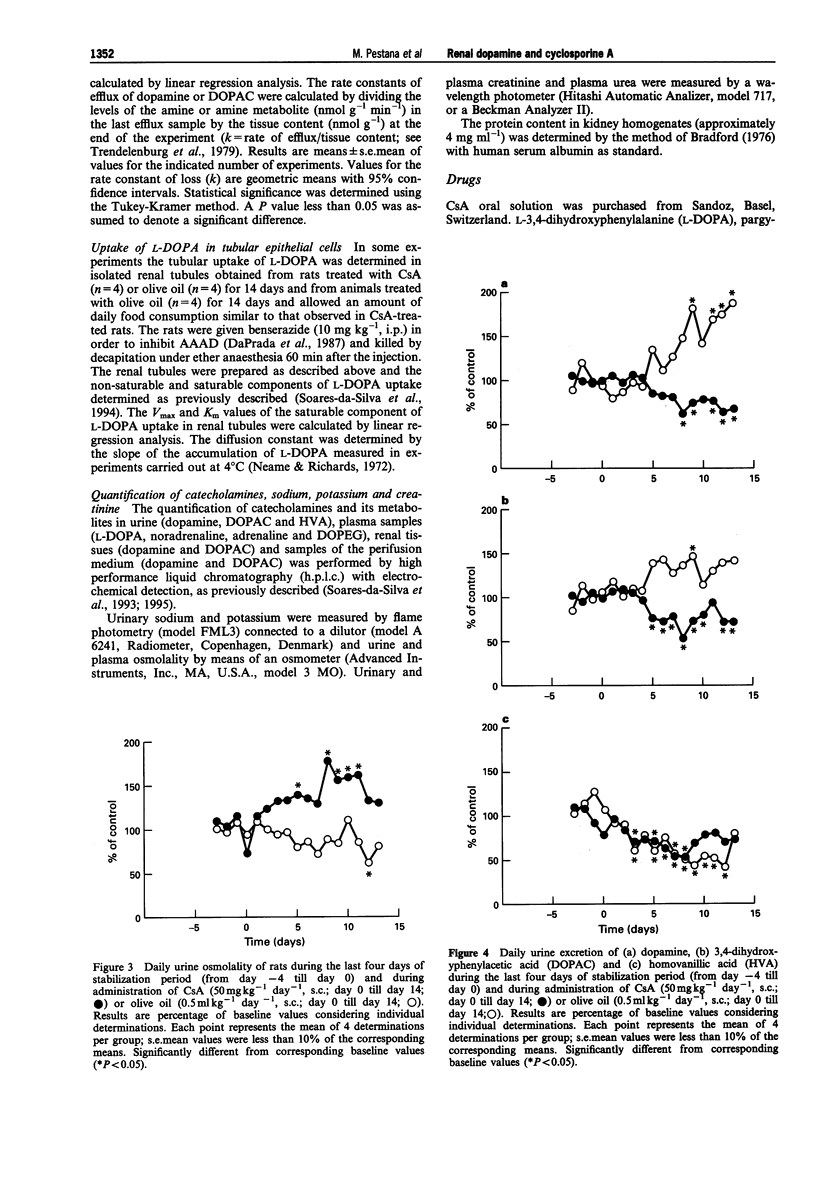
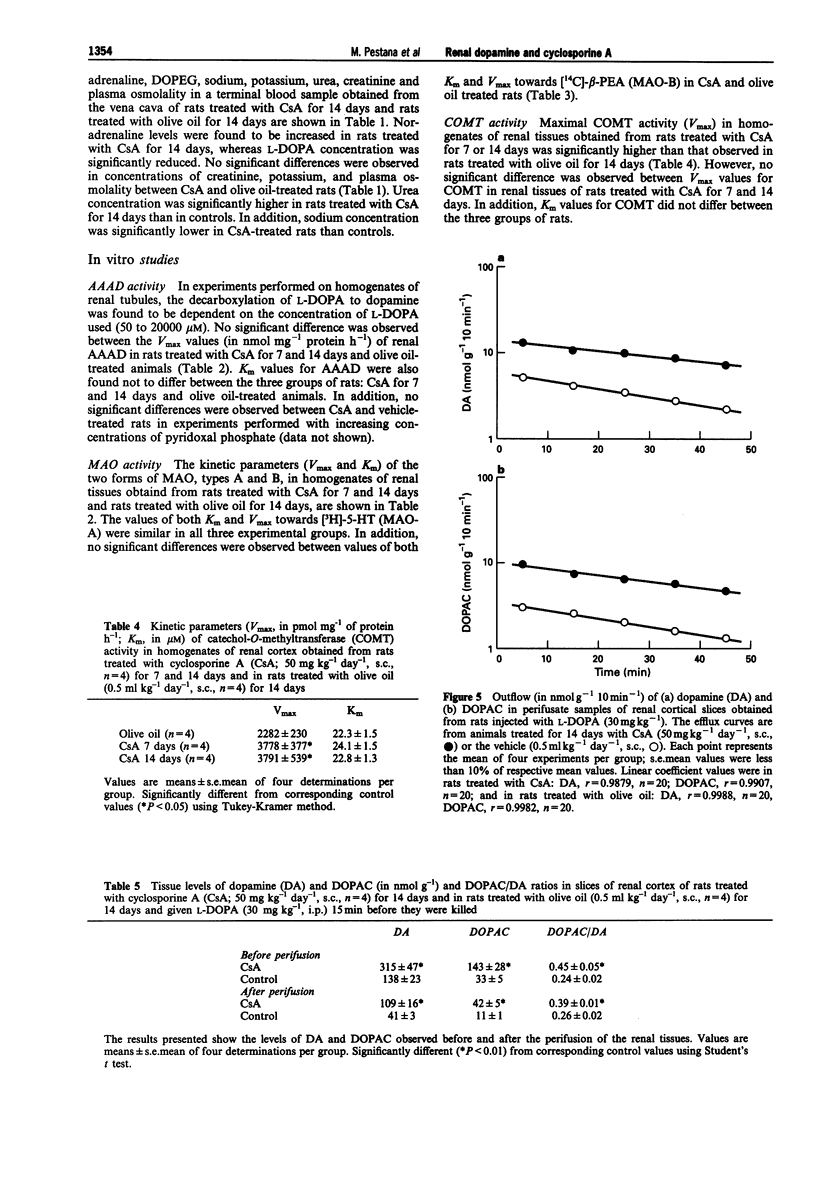
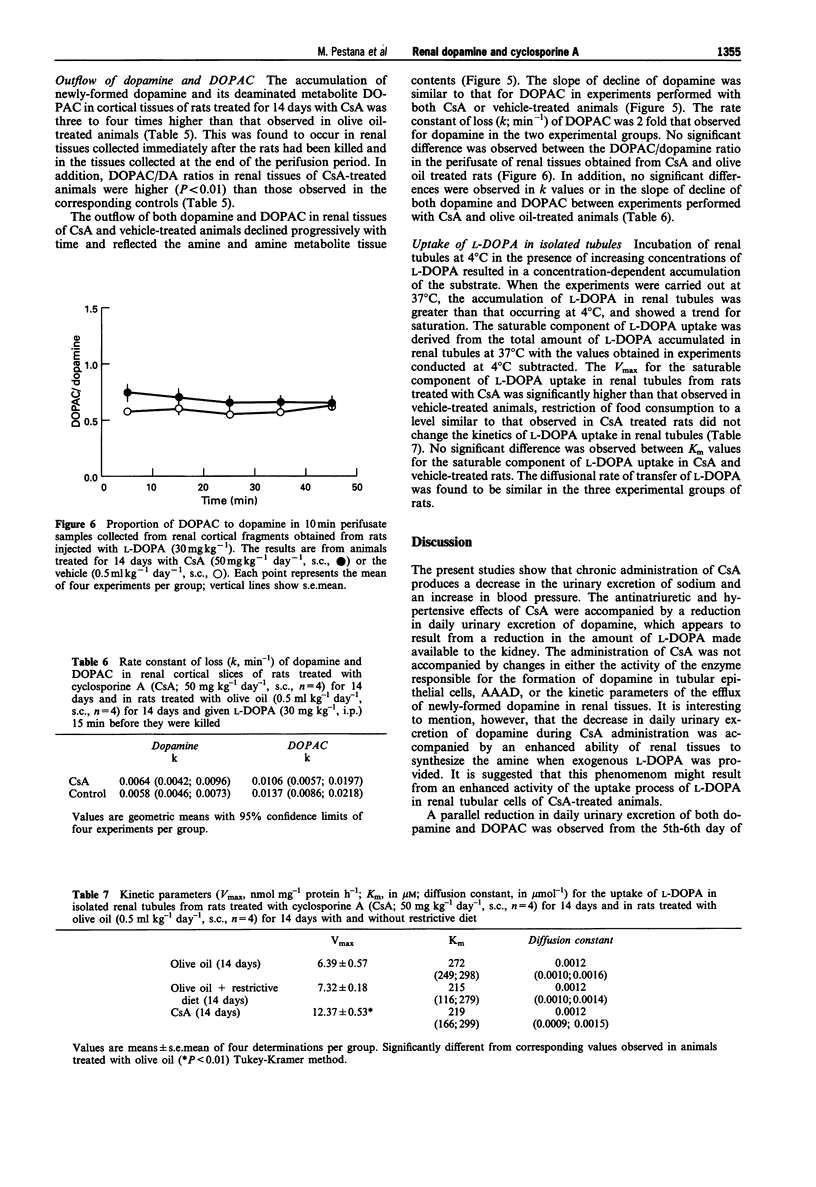
1. Administration of cyclosporine A (CsA; 50 mg kg-1 day-1, s.c.) for 14 days produced an increase in both systolic (SBP) and diastolic (DBP) blood pressure by 60 and 25 mmHg, respectively. The urinary excretion of dopamine, DOPAC and HVA was reduced from day 5-6 of CsA administration onwards (dopamine from 19 to 46%, DOPAC from 16 to 48%; HVA from 18 to 42%). In vehicle-treated rats, the urinary excretion of dopamine and DOPAC increased (from 7 to 60%) from day 5 onwards; by contrast, the urinary excretion of HVA was reduced (from 27 to 60%) during the second week. 2. No significant difference was observed between the Vmax and Km values of renal aromatic L-amino acid decarboxylase (AAAD) in rats treated with CsA for 7 and 14 days or with vehicle. 3. Km and Vmax of monoamine oxidase types A and B did not differ significantly between rats treated with CsA for 7 and 14 days or with vehicle. 4. Maximal catechol-O-methyltransferase activity (Vmax) in homogenates of renal tissues obtained from rats treated with CsA for 7 or 14 days was significantly higher than that in vehicle-treated rats; Km (22.3 +/- 1.5 microM) values for COMT did not differ between the three groups of rats. 5. The accumulation of newly-formed dopamine and DOPAC in cortical tissues of rats treated with CsA for 14 days was three to four times higher than in controls. The outflow of both dopamine and DOPAC declined progressively with time and reflected the amine and amine metabolite tissue contents. No significant difference was observed between the DOPAC/dopamine ratios in the perifusate of renal tissues obtained from CsA- and vehicle-treated rats. In addition, no significant differences were observed in k values or in the slope of decline of both DA and DOPAC between experiments performed with CsA and vehicle-treated animals. 6. The Vmax for the saturable component of L-3,4-dihydroxyphenylalanine (L-DOPA) uptake in renal tubules from rats treated with CsA was twice that of vehicle-treated animals. Km in CsA- and vehicle-treated rats did not differ. 7. The decrease in the urinary excretion of sodium and an increase in blood pressure during CsA treatment was accompanied by a reduction in daily urinary excretion of dopamine. This appears to result from a reduction in the amount of L-DOPA made available to the kidney and does not involve changes in tubular AAAD, the availability of dopamine to leave the renal cells and dopamine metabolism.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bertorello A., Hökfelt T., Goldstein M., Aperia A. Proximal tubule Na+-K+-ATPase activity is inhibited during high-salt diet: evidence for DA-mediated effect. Am J Physiol. 1988 Jun;254(6 Pt 2):F795–F801. doi: 10.1152/ajprenal.1988.254.6.F795. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brooks D. P., Drutz D. J., Ruffolo R. R., Jr Prevention and complete reversal of cyclosporine A-induced renal vasoconstriction and nephrotoxicity in the rat by fenoldopam. J Pharmacol Exp Ther. 1990 Aug;254(2):375–379. [PubMed] [Google Scholar]
- Conte G., Sabbatini M., Napodano P., De Nicola L., Gigliotti G., Fuiano G., Testa A., Russo D., Esposito C., Libetta C. Dopamine counteracts the acute renal effects of cyclosporine in normal subjects. Transplant Proc. 1988 Jun;20(3 Suppl 3):563–567. [PubMed] [Google Scholar]
- Da Prada M., Kettler R., Zürcher G., Schaffner R., Haefely W. E. Inhibition of decarboxylase and levels of dopa and 3-O-methyldopa: a comparative study of benserazide versus carbidopa in rodents and of Madopar standard versus Madopar HBS in volunteers. Eur Neurol. 1987;27 (Suppl 1):9–20. doi: 10.1159/000116170. [DOI] [PubMed] [Google Scholar]
- Dieperink H., Leyssac P. P., Starklint H., Kemp E. Long-term cyclosporin nephrotoxicity in the rat: effects on renal function and morphology. Nephrol Dial Transplant. 1988;3(3):317–326. [PubMed] [Google Scholar]
- Eisenhofer G., Brush J. E., Cannon R. O., 3rd, Stull R., Kopin I. J., Goldstein D. S. Plasma dihydroxyphenylalanine and total body and regional noradrenergic activity in humans. J Clin Endocrinol Metab. 1989 Feb;68(2):247–255. doi: 10.1210/jcem-68-2-247. [DOI] [PubMed] [Google Scholar]
- Felder R. A., Felder C. C., Eisner G. M., Jose P. A. The dopamine receptor in adult and maturing kidney. Am J Physiol. 1989 Sep;257(3 Pt 2):F315–F327. doi: 10.1152/ajprenal.1989.257.3.F315. [DOI] [PubMed] [Google Scholar]
- Fernandes M. H., Soares-da-Silva P. Type A and B monoamine oxidase activities in the human and rat kidney. Acta Physiol Scand. 1992 Aug;145(4):363–367. doi: 10.1111/j.1748-1716.1992.tb09376.x. [DOI] [PubMed] [Google Scholar]
- Goldstein D. S., Cannon R. O., 3rd, Quyyumi A., Chang P., Duncan M., Brush J. E., Jr, Eisenhofer G. Regional extraction of circulating norepinephrine, DOPA, and dihydroxyphenylglycol in humans. J Auton Nerv Syst. 1991 Jun 1;34(1):17–35. doi: 10.1016/0165-1838(91)90005-n. [DOI] [PubMed] [Google Scholar]
- Ishikawa A., Sakaguchi M., Nakano M., Ushiyama T., Ohta N., Ohtawara Y., Tajima A., Kawabe K., Aso Y. [Cyclosporine induced nephrotoxicity and juxtaglomerular apparatus in mice]. Nihon Hinyokika Gakkai Zasshi. 1991 Aug;82(8):1286–1291. doi: 10.5980/jpnjurol1989.82.1286. [DOI] [PubMed] [Google Scholar]
- Kaskel F. J., Devarajan P., Arbeit L. A., Partin J. S., Moore L. C. Cyclosporine nephrotoxicity: sodium excretion, autoregulation, and angiotensin II. Am J Physiol. 1987 Apr;252(4 Pt 2):F733–F742. doi: 10.1152/ajprenal.1987.252.4.F733. [DOI] [PubMed] [Google Scholar]
- Kon V., Sugiura M., Inagami T., Harvie B. R., Ichikawa I., Hoover R. L. Role of endothelin in cyclosporine-induced glomerular dysfunction. Kidney Int. 1990 Jun;37(6):1487–1491. doi: 10.1038/ki.1990.139. [DOI] [PubMed] [Google Scholar]
- Kopin I. J. Catecholamine metabolism: basic aspects and clinical significance. Pharmacol Rev. 1985 Dec;37(4):333–364. [PubMed] [Google Scholar]
- Kopp J. B., Klotman P. E. Cellular and molecular mechanisms of cyclosporin nephrotoxicity. J Am Soc Nephrol. 1990 Aug;1(2):162–179. doi: 10.1681/ASN.V12162. [DOI] [PubMed] [Google Scholar]
- Lee M. R. Dopamine and the kidney: ten years on. Clin Sci (Lond) 1993 Apr;84(4):357–375. doi: 10.1042/cs0840357. [DOI] [PubMed] [Google Scholar]
- Pestana M., Soares-da-Silva P. The renal handling of dopamine originating from L-dopa and gamma-glutamyl-L-dopa. Br J Pharmacol. 1994 Jun;112(2):417–422. doi: 10.1111/j.1476-5381.1994.tb13088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petric R., Freeman D., Wallace C., McDonald J., Stiller C., Keown P. Modulation of experimental cyclosporine nephrotoxicity by inhibition of thromboxane synthesis. Transplantation. 1990 Oct;50(4):558–563. doi: 10.1097/00007890-199010000-00005. [DOI] [PubMed] [Google Scholar]
- Remuzzi G., Bertani T. Renal vascular and thrombotic effects of cyclosporine. Am J Kidney Dis. 1989 Apr;13(4):261–272. doi: 10.1016/s0272-6386(89)80032-0. [DOI] [PubMed] [Google Scholar]
- Sabbatini M., Esposito C., De Nicola L., Uccello F., Altomonte M., Conte G., Dal Canton A., Andreucci V. E. Reversibility of acute cyclosporin nephrotoxicity by dopamine. Micropuncture study in the rat. Nephrol Dial Transplant. 1989;4(5):327–333. doi: 10.1093/oxfordjournals.ndt.a091885. [DOI] [PubMed] [Google Scholar]
- Siegl H., Ryffel B. Effect of cyclosporin on renin-angiotensin-aldosterone system. Lancet. 1982 Dec 4;2(8310):1274–1274. doi: 10.1016/s0140-6736(82)90124-6. [DOI] [PubMed] [Google Scholar]
- Siragy H. M., Felder R. A., Howell N. L., Chevalier R. L., Peach M. J., Carey R. M. Evidence that intrarenal dopamine acts as a paracrine substance at the renal tubule. Am J Physiol. 1989 Sep;257(3 Pt 2):F469–F477. doi: 10.1152/ajprenal.1989.257.3.F469. [DOI] [PubMed] [Google Scholar]
- Soares-da-Silva P., Fernandes M. H., Pestana M. Studies on the role of sodium on the synthesis of dopamine in the rat kidney. J Pharmacol Exp Ther. 1993 Jan;264(1):406–414. [PubMed] [Google Scholar]
- Soares-da-Silva P., Fernandes M. H., Pinto-do-O P. C. Cell inward transport of L-DOPA and 3-O-methyl-L-DOPA in rat renal tubules. Br J Pharmacol. 1994 Jun;112(2):611–615. doi: 10.1111/j.1476-5381.1994.tb13118.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares-da-Silva P., Pestana M., Vieira-Coelho M. A., Fernandes M. H., Albino-Teixeira A. Assessment of renal dopaminergic system activity in the nitric oxide-deprived hypertensive rat model. Br J Pharmacol. 1995 Apr;114(7):1403–1413. doi: 10.1111/j.1476-5381.1995.tb13362.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturrock N. D., Struthers A. D. Hormonal and other mechanisms involved in the pathogenesis of cyclosporin-induced nephrotoxicity and hypertension in man. Clin Sci (Lond) 1994 Jan;86(1):1–9. doi: 10.1042/cs0860001. [DOI] [PubMed] [Google Scholar]
- Trendelenburg U., Bönisch H., Graefe K. H., Henseling M. The rate constants for the efflux of metabolites of catecholamines and phenethylamines. Pharmacol Rev. 1979 Sep;31(3):179–203. [PubMed] [Google Scholar]
- Túri S., Beattie T. J., Belch J. J., Murphy A. V. Disturbances of prostacyclin metabolism in children with hemolytic-uremic syndrome and in first degree relatives. Clin Nephrol. 1986 Apr;25(4):193–198. [PubMed] [Google Scholar]
- Van Loon G. R. Plasma dopamine: regulation and significance. Fed Proc. 1983 Oct;42(13):3012–3018. [PubMed] [Google Scholar]
- Vieira-Coelho M. A., Fernandes M. H., Soares-da-Silva P. In vivo effects of the monoamine oxidase inhibitors Ro 41-1049 and Ro 19-6327 on the production and fate of renal dopamine. J Neural Transm Suppl. 1994;41:365–370. doi: 10.1007/978-3-7091-9324-2_48. [DOI] [PubMed] [Google Scholar]
- Whiting P. H., Propper D. J., Simpson J. G., McKay J., Jones M. C., Catto G. R. The use of lithium clearance measurements to assess renal tubular function in experimental and clinical cyclosporine nephrotoxicity. Transplant Proc. 1988 Jun;20(3 Suppl 3):675–680. [PubMed] [Google Scholar]
- Wolfovitz E., Grossman E., Folio C. J., Keiser H. R., Kopin I. J., Goldstein D. S. Derivation of urinary dopamine from plasma dihydroxyphenylalanine in humans. Clin Sci (Lond) 1993 May;84(5):549–557. doi: 10.1042/cs0840549. [DOI] [PubMed] [Google Scholar]



