Abstract
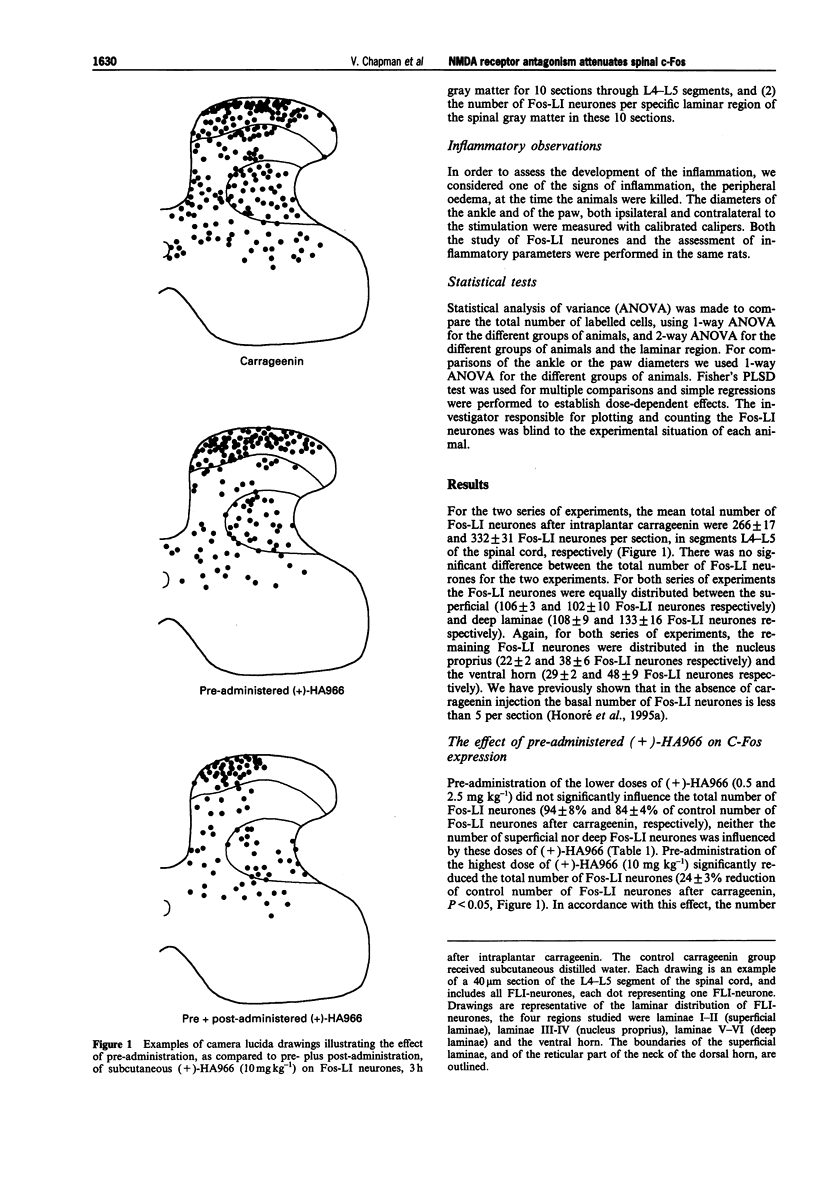
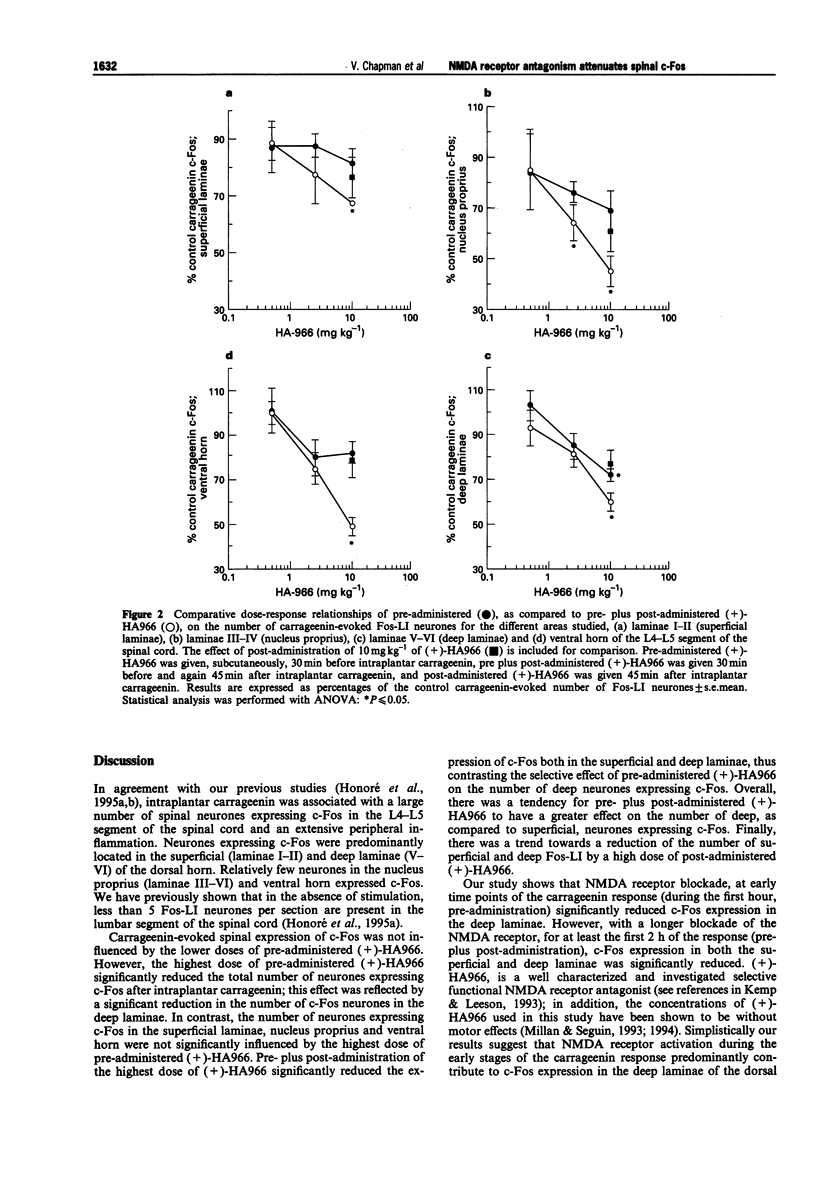
1. Intraplantar carrageenin (6 mg 150 microliters-1) evoked a high level of spinal c-Fos expression in the dorsal horn, of segments L4-L5 of the spinal cord, and an extensive peripheral oedema; both parameters were assessed 3 h after carrageenin. 2. Two series of experiments were performed, with the mean total number of Fos like-immunoreactive neurones (Fos-LI), after carrageenin, not being significantly different for the two series of experiments (266 +/- 17 and 332 +/- 31 Fos-LI neurones). For both series of experiments Fos-LI neurones were predominantly located in the superficial and deep laminae, only 10% of the total number of Fos-LI neurones were located in the nucleus proprius and 10% were located in the ventral horn. 3. Pre-administration of the N-methyl-D-aspartate (NMDA) receptor antagonist, (+)-HA966 (0.5 mg kg-1 and 2.5 mg kg-1, s.c.), 30 min before carrageenin, did not significantly influence the total number of Fos-LI neurones, as compared to control carrageenin expression. 4. Pre-administration of the highest dose of (+)-HA966 (10 mg kg-1) significantly reduced the number of deep laminae Fos-LI neurones (28 +/- 3% reduction of control number of Fos-LI neurones after carrageenin, P < or = 0.05), without influencing the number of superficial Fos-LI neurones. There was a tendency towards a reduction of the number of Fos-LI neurones in the nucleus proprius by the highest concentration of pre-administered (+)-HA966, (31 +/- 8% reduction), but this effect did not reach significance.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chapman V., Dickenson A. H. Enhanced responses of rat dorsal horn neurons after UV irradiation of the hindpaw; roles of the NMDA receptor. Neurosci Lett. 1994 Jul 18;176(1):41–44. doi: 10.1016/0304-3940(94)90866-4. [DOI] [PubMed] [Google Scholar]
- Coderre T. J., Melzack R. The contribution of excitatory amino acids to central sensitization and persistent nociception after formalin-induced tissue injury. J Neurosci. 1992 Sep;12(9):3665–3670. doi: 10.1523/JNEUROSCI.12-09-03665.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickenson A. H., Aydar E. Antagonism at the glycine site on the NMDA receptor reduces spinal nociception in the rat. Neurosci Lett. 1991 Jan 2;121(1-2):263–266. doi: 10.1016/0304-3940(91)90700-4. [DOI] [PubMed] [Google Scholar]
- Dickenson A. H., Sullivan A. F. Differential effects of excitatory amino acid antagonists on dorsal horn nociceptive neurones in the rat. Brain Res. 1990 Jan 1;506(1):31–39. doi: 10.1016/0006-8993(90)91195-m. [DOI] [PubMed] [Google Scholar]
- Draisci G., Iadarola M. J. Temporal analysis of increases in c-fos, preprodynorphin and preproenkephalin mRNAs in rat spinal cord. Brain Res Mol Brain Res. 1989 Jul;6(1):31–37. doi: 10.1016/0169-328x(89)90025-9. [DOI] [PubMed] [Google Scholar]
- Dubner R., Ruda M. A. Activity-dependent neuronal plasticity following tissue injury and inflammation. Trends Neurosci. 1992 Mar;15(3):96–103. doi: 10.1016/0166-2236(92)90019-5. [DOI] [PubMed] [Google Scholar]
- Eisenberg E., LaCross S., Strassman A. M. The effects of the clinically tested NMDA receptor antagonist memantine on carrageenan-induced thermal hyperalgesia in rats. Eur J Pharmacol. 1994 Apr 1;255(1-3):123–129. doi: 10.1016/0014-2999(94)90090-6. [DOI] [PubMed] [Google Scholar]
- Ferreira S. H., Lorenzetti B. B., Poole S. Bradykinin initiates cytokine-mediated inflammatory hyperalgesia. Br J Pharmacol. 1993 Nov;110(3):1227–1231. doi: 10.1111/j.1476-5381.1993.tb13946.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haley J. E., Sullivan A. F., Dickenson A. H. Evidence for spinal N-methyl-D-aspartate receptor involvement in prolonged chemical nociception in the rat. Brain Res. 1990 Jun 4;518(1-2):218–226. doi: 10.1016/0006-8993(90)90975-h. [DOI] [PubMed] [Google Scholar]
- Herdegen T., Kovary K., Leah J., Bravo R. Specific temporal and spatial distribution of JUN, FOS, and KROX-24 proteins in spinal neurons following noxious transsynaptic stimulation. J Comp Neurol. 1991 Nov 1;313(1):178–191. doi: 10.1002/cne.903130113. [DOI] [PubMed] [Google Scholar]
- Honoré P., Buritova J., Besson J. M. Carrageenin-evoked c-Fos expression in rat lumbar spinal cord: the effects of indomethacin. Eur J Pharmacol. 1995 Jan 16;272(2-3):249–259. doi: 10.1016/0014-2999(94)00656-r. [DOI] [PubMed] [Google Scholar]
- Honoré P., Chapman V., Buritova J., Besson J. M. Reduction of carrageenin oedema and the associated c-Fos expression in the rat lumbar spinal cord by nitric oxide synthase inhibitor. Br J Pharmacol. 1995 Jan;114(1):77–84. doi: 10.1111/j.1476-5381.1995.tb14908.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu S. M., Raine L., Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem. 1981 Apr;29(4):577–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- Hunter J. C., Singh L. Role of excitatory amino acid receptors in the mediation of the nociceptive response to formalin in the rat. Neurosci Lett. 1994 Jun 20;174(2):217–221. doi: 10.1016/0304-3940(94)90025-6. [DOI] [PubMed] [Google Scholar]
- Joris J., Costello A., Dubner R., Hargreaves K. M. Opiates suppress carrageenan-induced edema and hyperthermia at doses that inhibit hyperalgesia. Pain. 1990 Oct;43(1):95–103. doi: 10.1016/0304-3959(90)90054-H. [DOI] [PubMed] [Google Scholar]
- Kemp J. A., Leeson P. D. The glycine site of the NMDA receptor--five years on. Trends Pharmacol Sci. 1993 Jan;14(1):20–25. doi: 10.1016/0165-6147(93)90108-v. [DOI] [PubMed] [Google Scholar]
- Kocher L., Anton F., Reeh P. W., Handwerker H. O. The effect of carrageenan-induced inflammation on the sensitivity of unmyelinated skin nociceptors in the rat. Pain. 1987 Jun;29(3):363–373. doi: 10.1016/0304-3959(87)90051-0. [DOI] [PubMed] [Google Scholar]
- Kristensen J. D., Karlsten R., Gordh T., Berge O. G. The NMDA antagonist 3-(2-carboxypiperazin-4-yl)propyl-1-phosphonic acid (CPP) has antinociceptive effect after intrathecal injection in the rat. Pain. 1994 Jan;56(1):59–67. doi: 10.1016/0304-3959(94)90150-3. [DOI] [PubMed] [Google Scholar]
- Malmberg A. B., Yaksh T. L. Voltage-sensitive calcium channels in spinal nociceptive processing: blockade of N- and P-type channels inhibits formalin-induced nociception. J Neurosci. 1994 Aug;14(8):4882–4890. doi: 10.1523/JNEUROSCI.14-08-04882.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer M. L., Westbrook G. L. Permeation and block of N-methyl-D-aspartic acid receptor channels by divalent cations in mouse cultured central neurones. J Physiol. 1987 Dec;394:501–527. doi: 10.1113/jphysiol.1987.sp016883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millan M. J., Seguin L. (+)-HA 966, a partial agonist at the glycine site coupled to NMDA receptors, blocks formalin-induced pain in mice. Eur J Pharmacol. 1993 Jul 20;238(2-3):445–447. doi: 10.1016/0014-2999(93)90884-k. [DOI] [PubMed] [Google Scholar]
- Millan M. J., Seguin L. Chemically-diverse ligands at the glycine B site coupled to N-methyl-D-aspartate (NMDA) receptors selectively block the late phase of formalin-induced pain in mice. Neurosci Lett. 1994 Aug 29;178(1):139–143. doi: 10.1016/0304-3940(94)90309-3. [DOI] [PubMed] [Google Scholar]
- Molander C., Xu Q., Grant G. The cytoarchitectonic organization of the spinal cord in the rat. I. The lower thoracic and lumbosacral cord. J Comp Neurol. 1984 Nov 20;230(1):133–141. doi: 10.1002/cne.902300112. [DOI] [PubMed] [Google Scholar]
- Morgan J. I., Curran T. Immediate-early genes: ten years on. Trends Neurosci. 1995 Feb;18(2):66–67. [PubMed] [Google Scholar]
- Noguchi K., Dubner R., Ruda M. A. Preproenkephalin mRNA in spinal dorsal horn neurons is induced by peripheral inflammation and is co-localized with Fos and Fos-related proteins. Neuroscience. 1992;46(3):561–570. doi: 10.1016/0306-4522(92)90144-q. [DOI] [PubMed] [Google Scholar]
- Noguchi K., Kowalski K., Traub R., Solodkin A., Iadarola M. J., Ruda M. A. Dynorphin expression and Fos-like immunoreactivity following inflammation induced hyperalgesia are colocalized in spinal cord neurons. Brain Res Mol Brain Res. 1991 Jun;10(3):227–233. doi: 10.1016/0169-328x(91)90065-6. [DOI] [PubMed] [Google Scholar]
- Price D. D., Mao J., Frenk H., Mayer D. J. The N-methyl-D-aspartate receptor antagonist dextromethorphan selectively reduces temporal summation of second pain in man. Pain. 1994 Nov;59(2):165–174. doi: 10.1016/0304-3959(94)90069-8. [DOI] [PubMed] [Google Scholar]
- Ren K., Williams G. M., Hylden J. L., Ruda M. A., Dubner R. The intrathecal administration of excitatory amino acid receptor antagonists selectively attenuated carrageenan-induced behavioral hyperalgesia in rats. Eur J Pharmacol. 1992 Aug 25;219(2):235–243. doi: 10.1016/0014-2999(92)90301-j. [DOI] [PubMed] [Google Scholar]
- Thompson S. W., Dray A., Urban L. Injury-induced plasticity of spinal reflex activity: NK1 neurokinin receptor activation and enhanced A- and C-fiber mediated responses in the rat spinal cord in vitro. J Neurosci. 1994 Jun;14(6):3672–3687. doi: 10.1523/JNEUROSCI.14-06-03672.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban L., Thompson S. W., Dray A. Modulation of spinal excitability: co-operation between neurokinin and excitatory amino acid neurotransmitters. Trends Neurosci. 1994 Oct;17(10):432–438. doi: 10.1016/0166-2236(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Vaccarino A. L., Marek P., Kest B., Weber E., Keana J. F., Liebeskind J. C. NMDA receptor antagonists, MK-801 and ACEA-1011, prevent the development of tonic pain following subcutaneous formalin. Brain Res. 1993 Jul 2;615(2):331–334. doi: 10.1016/0006-8993(93)90045-o. [DOI] [PubMed] [Google Scholar]
- WINTER C. A., RISLEY E. A., NUSS G. W. Carrageenin-induced edema in hind paw of the rat as an assay for antiiflammatory drugs. Proc Soc Exp Biol Med. 1962 Dec;111:544–547. doi: 10.3181/00379727-111-27849. [DOI] [PubMed] [Google Scholar]
- Woolf C. J., Doubell T. P. The pathophysiology of chronic pain--increased sensitivity to low threshold A beta-fibre inputs. Curr Opin Neurobiol. 1994 Aug;4(4):525–534. doi: 10.1016/0959-4388(94)90053-1. [DOI] [PubMed] [Google Scholar]
- Yaksh T. L. Behavioral and autonomic correlates of the tactile evoked allodynia produced by spinal glycine inhibition: effects of modulatory receptor systems and excitatory amino acid antagonists. Pain. 1989 Apr;37(1):111–123. doi: 10.1016/0304-3959(89)90160-7. [DOI] [PubMed] [Google Scholar]
- Yamamoto T., Yaksh T. L. Comparison of the antinociceptive effects of pre- and posttreatment with intrathecal morphine and MK801, an NMDA antagonist, on the formalin test in the rat. Anesthesiology. 1992 Oct;77(4):757–763. doi: 10.1097/00000542-199210000-00021. [DOI] [PubMed] [Google Scholar]


