Abstract
1. Non-adrenergic, non-cholinergic (NANC) relaxations induced by electrical field stimulation (EFS) were studied in pig isolated urethra. The mechanism for relaxation was characterized by measurement of cyclic nucleotides and by study of involvement of different subsets of voltage-operated calcium channels (VOCCs). 2. EFS evoked frequency-dependent and tetrodotoxin-sensitive relaxations in the presence of propranolol (1 microM), phentolamine (1 microM) and scopolamine (1 microM). At low frequencies (< 12 Hz), relaxations were rapid, whereas at high (> 12 Hz) frequencies distinct biphasic relaxations were evoked. The latter consisted of a rapidly developing first phase followed by a more long-lasting second phase. 3. Treatment with the NO-synthesis inhibitor NG-nitro-L-arginine (L-NOARG; 0.3 mM) inhibited relaxations at low frequencies of stimulation. At high frequencies (> 12 Hz) only the first relaxation phase was affected. 4. Measurement of cyclic nucleotides in preparations subjected to continuous nerve-stimulation, revealed an increase in guanosine 3':5'-cyclic monophosphate (cyclic GMP) levels from 1.3 +/- 0.3 to 3.0 +/- 0.4 pmol mg-1 protein (P < 0.01). In the presence of L-NOARG, there was a significant decrease in cyclic GMP content to control. However, there was no increase in cyclic GMP content in response to EFS. Levels of cyclic AMP remained unchanged following EFS. 5. Treatment with the N-type VOCC-inhibitor, omega-conotoxin GVIA (0.1 microM) reduced NO-dependent relaxations, the effect being most pronounced at low frequencies (1-4 Hz) of stimulation.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF

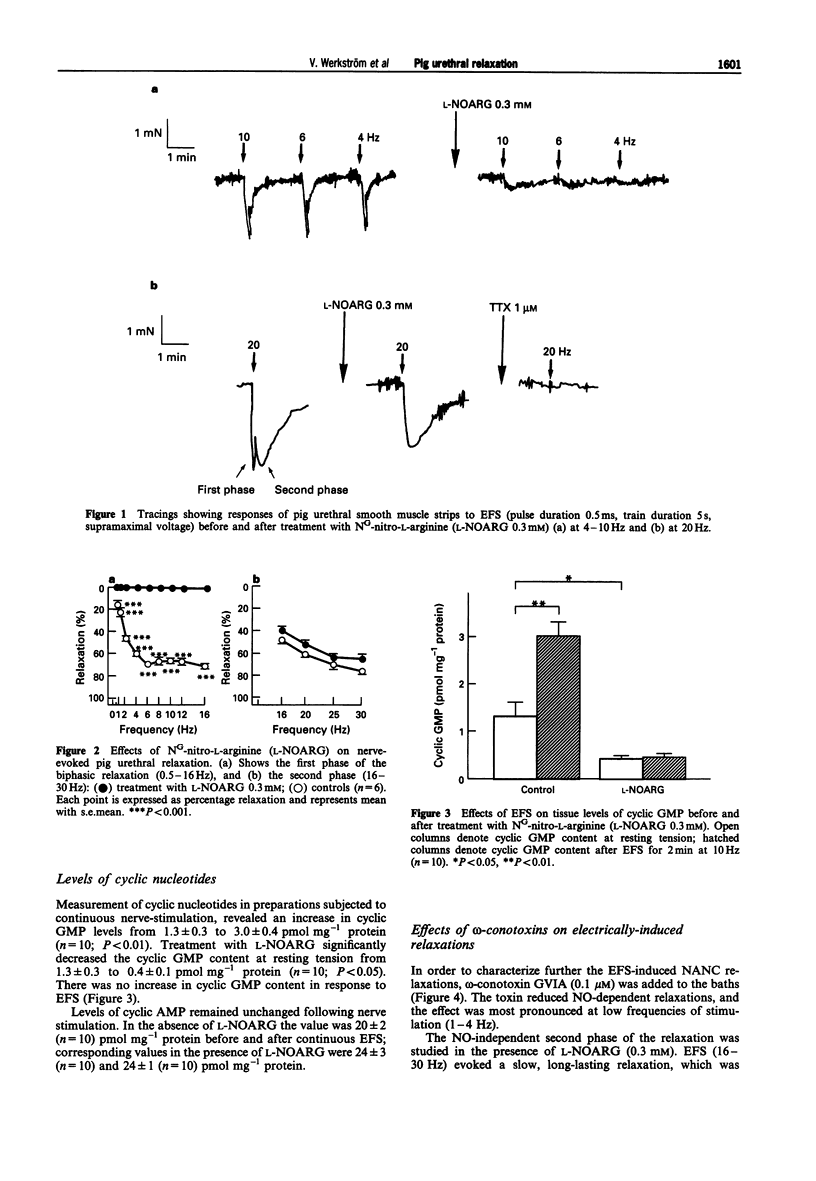


Selected References
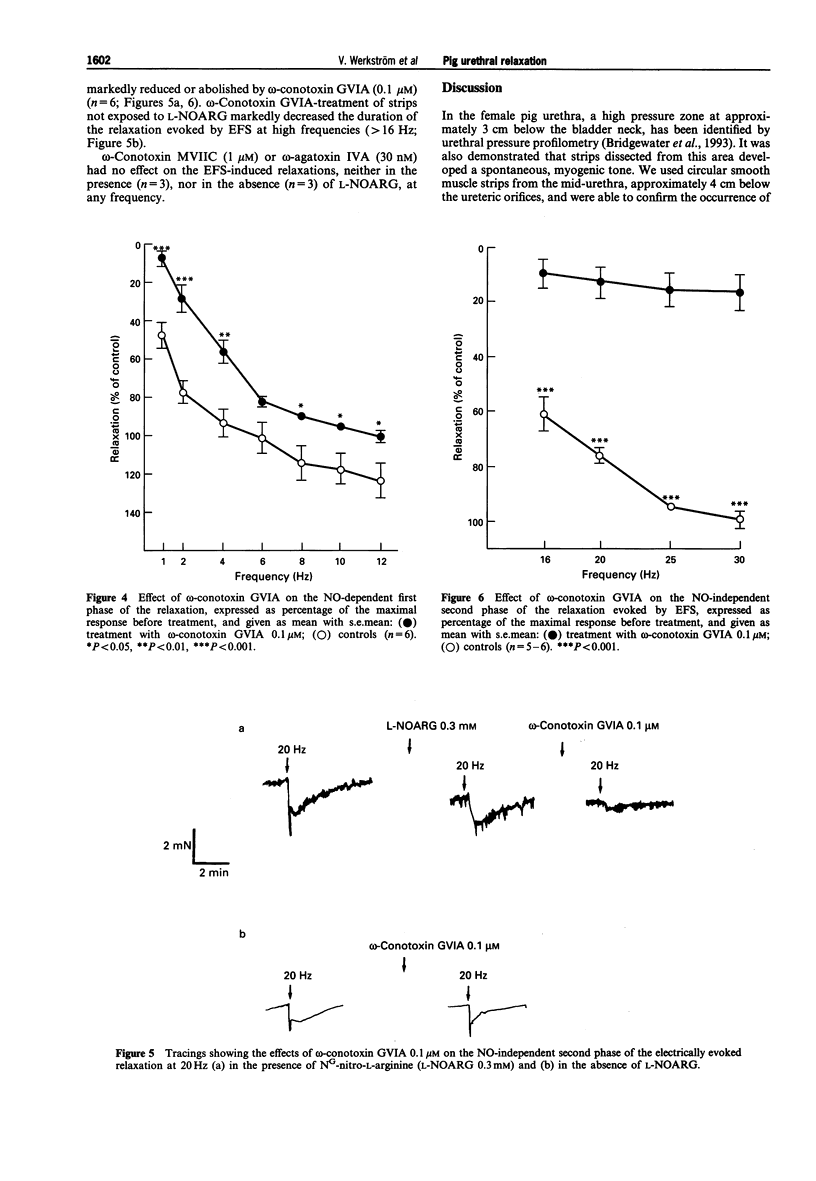
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson K. E. Pharmacology of lower urinary tract smooth muscles and penile erectile tissues. Pharmacol Rev. 1993 Sep;45(3):253–308. [PubMed] [Google Scholar]
- Andersson K. E., Persson K. Nitric oxide synthase and nitric oxide-mediated effects in lower urinary tract smooth muscles. World J Urol. 1994;12(5):274–280. doi: 10.1007/BF00191207. [DOI] [PubMed] [Google Scholar]
- Belai A., Ralevic V., Burnstock G. VIP release from enteric nerves is independent of extracellular calcium. Regul Pept. 1987 Oct;19(1-2):79–89. doi: 10.1016/0167-0115(87)90077-2. [DOI] [PubMed] [Google Scholar]
- Boeckxstaens G. E., De Man J. G., Pelckmans P. A., Cromheeke K. M., Herman A. G., Van Maercke Y. M. Ca2+ dependency of the release of nitric oxide from non-adrenergic non-cholinergic nerves. Br J Pharmacol. 1993 Dec;110(4):1329–1334. doi: 10.1111/j.1476-5381.1993.tb13964.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeckxstaens G. E., Pelckmans P. A., De Man J. G., Bult H., Herman A. G., Van Maercke Y. M. Evidence for a differential release of nitric oxide and vasoactive intestinal polypeptide by nonadrenergic noncholinergic nerves in the rat gastric fundus. Arch Int Pharmacodyn Ther. 1992 Jul-Aug;318:107–115. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bridgewater M., MacNeil H. F., Brading A. F. Regulation of tone in pig urethral smooth muscle. J Urol. 1993 Jul;150(1):223–228. doi: 10.1016/s0022-5347(17)35451-4. [DOI] [PubMed] [Google Scholar]
- Dokita S., Smith S. D., Nishimoto T., Wheeler M. A., Weiss R. M. Involvement of nitric oxide and cyclic GMP in rabbit urethral relaxation. Eur J Pharmacol. 1994 Feb 15;266(3):269–275. doi: 10.1016/0922-4106(94)90136-8. [DOI] [PubMed] [Google Scholar]
- García-Pascual A., Triguero D. Relaxation mechanisms induced by stimulation of nerves and by nitric oxide in sheep urethral muscle. J Physiol. 1994 Apr 15;476(2):333–347. doi: 10.1113/jphysiol.1994.sp020135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto S., Kigoshi S., Muramatsu I. Neurogenic responses of urethra isolated from the dog. Eur J Pharmacol. 1992 Mar 17;213(1):117–123. doi: 10.1016/0014-2999(92)90240-5. [DOI] [PubMed] [Google Scholar]
- Hashimoto S., Kigoshi S., Muramatsu I. Nitric oxide-dependent and -independent neurogenic relaxation of isolated dog urethra. Eur J Pharmacol. 1993 Feb 9;231(2):209–214. doi: 10.1016/0014-2999(93)90451-m. [DOI] [PubMed] [Google Scholar]
- Hills J., Meldrum L. A., Klarskov P., Burnstock G. A novel non-adrenergic, non-cholinergic nerve-mediated relaxation of the pig bladder neck: an examination of possible neurotransmitter candidates. Eur J Pharmacol. 1984 Apr 6;99(4):287–293. doi: 10.1016/0014-2999(84)90135-3. [DOI] [PubMed] [Google Scholar]
- Hillyard D. R., Monje V. D., Mintz I. M., Bean B. P., Nadasdi L., Ramachandran J., Miljanich G., Azimi-Zoonooz A., McIntosh J. M., Cruz L. J. A new Conus peptide ligand for mammalian presynaptic Ca2+ channels. Neuron. 1992 Jul;9(1):69–77. doi: 10.1016/0896-6273(92)90221-x. [DOI] [PubMed] [Google Scholar]
- Keef K. D., Shuttleworth C. W., Xue C., Bayguinov O., Publicover N. G., Sanders K. M. Relationship between nitric oxide and vasoactive intestinal polypeptide in enteric inhibitory neurotransmission. Neuropharmacology. 1994 Nov;33(11):1303–1314. doi: 10.1016/0028-3908(94)90030-2. [DOI] [PubMed] [Google Scholar]
- Mintz I. M., Venema V. J., Swiderek K. M., Lee T. D., Bean B. P., Adams M. E. P-type calcium channels blocked by the spider toxin omega-Aga-IVA. Nature. 1992 Feb 27;355(6363):827–829. doi: 10.1038/355827a0. [DOI] [PubMed] [Google Scholar]
- Olivera B. M., Gray W. R., Zeikus R., McIntosh J. M., Varga J., Rivier J., de Santos V., Cruz L. J. Peptide neurotoxins from fish-hunting cone snails. Science. 1985 Dec 20;230(4732):1338–1343. doi: 10.1126/science.4071055. [DOI] [PubMed] [Google Scholar]
- Persson K., Alm P., Johansson K., Larsson B., Andersson K. E. Co-existence of nitrergic, peptidergic and acetylcholine esterase-positive nerves in the pig lower urinary tract. J Auton Nerv Syst. 1995 Apr 8;52(2-3):225–236. doi: 10.1016/0165-1838(94)00160-l. [DOI] [PubMed] [Google Scholar]
- Persson K., Andersson K. E. Non-adrenergic, non-cholinergic relaxation and levels of cyclic nucleotides in rabbit lower urinary tract. Eur J Pharmacol. 1994 Jul 15;268(2):159–167. doi: 10.1016/0922-4106(94)90185-6. [DOI] [PubMed] [Google Scholar]
- Snyder S. H. Nitric oxide: first in a new class of neurotransmitters. Science. 1992 Jul 24;257(5069):494–496. doi: 10.1126/science.1353273. [DOI] [PubMed] [Google Scholar]
- Tanagho E. A., Miller E. R. Initiation of voiding. Br J Urol. 1970 Apr;42(2):175–183. doi: 10.1111/j.1464-410x.1970.tb10019.x. [DOI] [PubMed] [Google Scholar]
- Zygmunt P. M., Zygmunt P. K., Högestätt E. D., Andersson K. E. Effects of omega-conotoxin on adrenergic, cholinergic and NANC neurotransmission in the rabbit urethra and detrusor. Br J Pharmacol. 1993 Dec;110(4):1285–1290. doi: 10.1111/j.1476-5381.1993.tb13957.x. [DOI] [PMC free article] [PubMed] [Google Scholar]



