Abstract
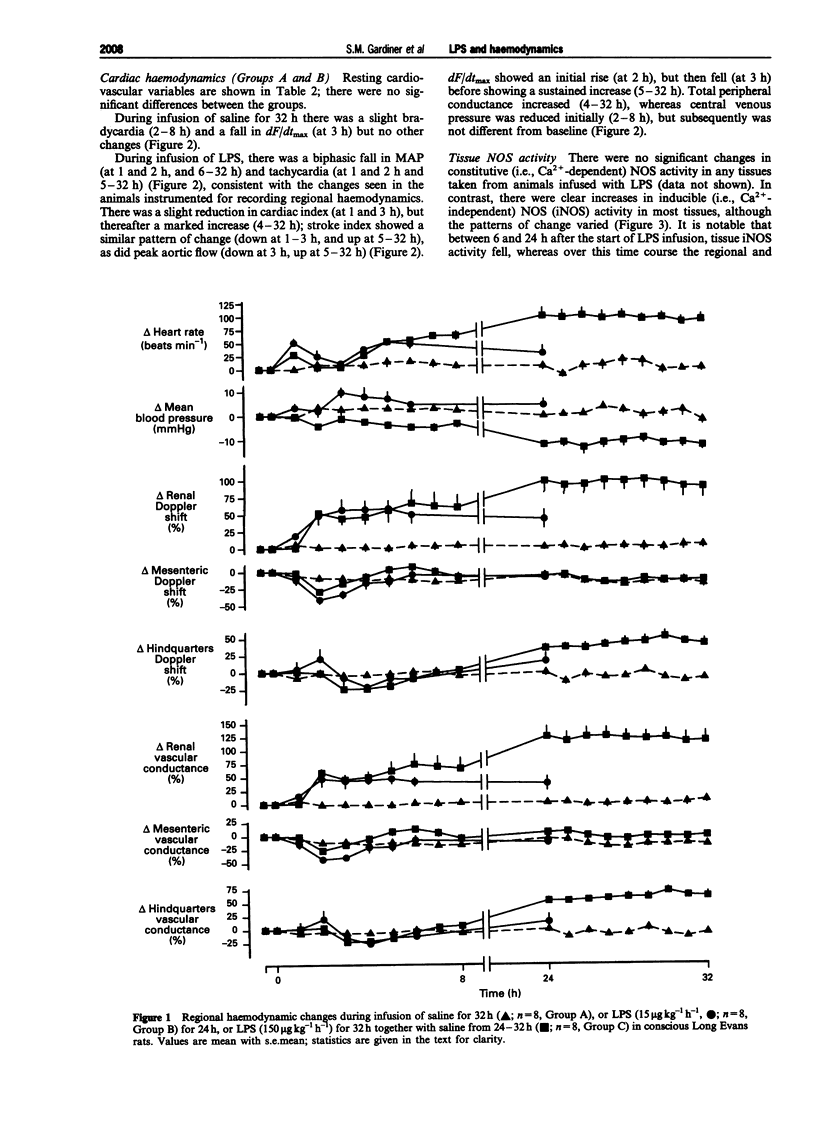
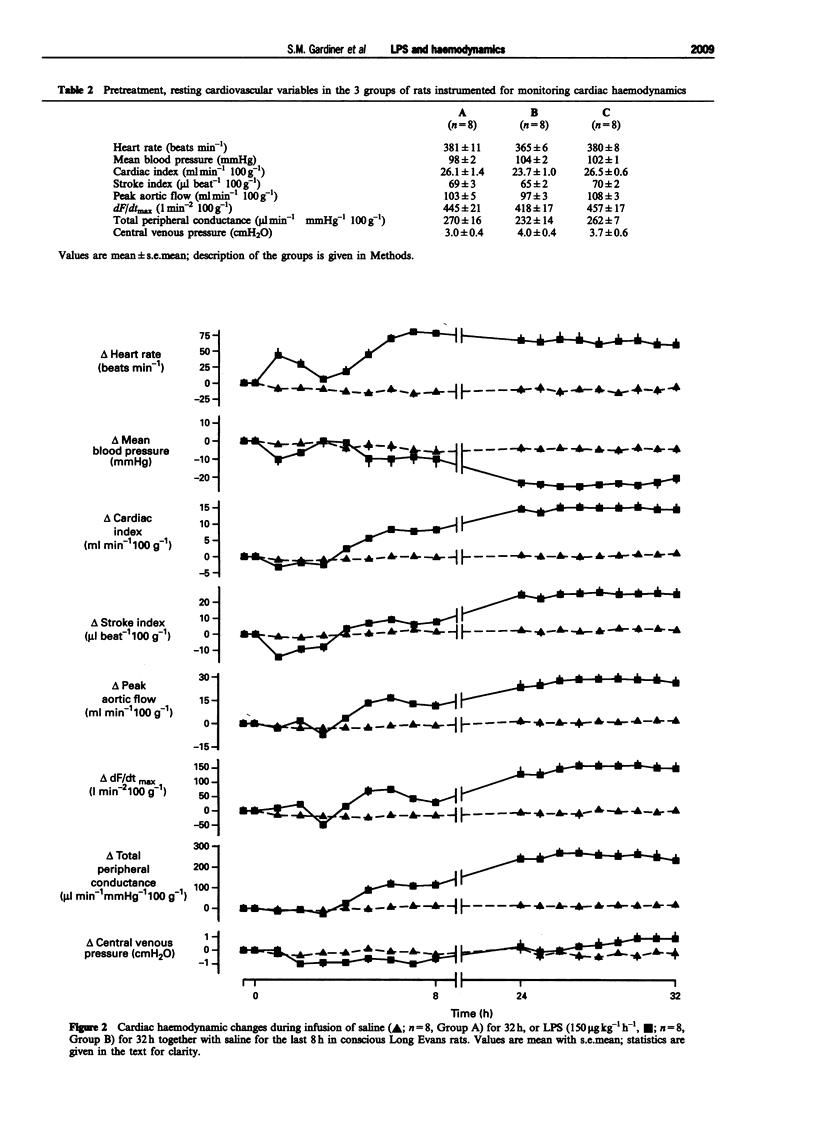
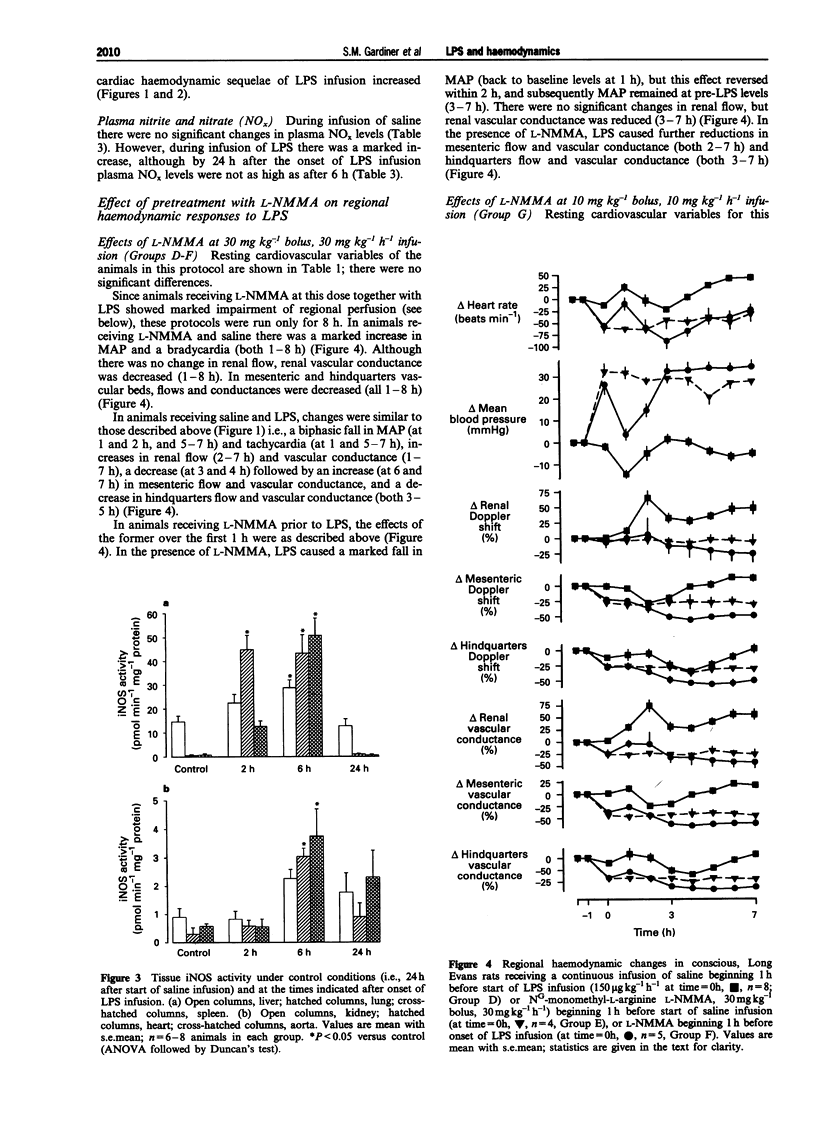
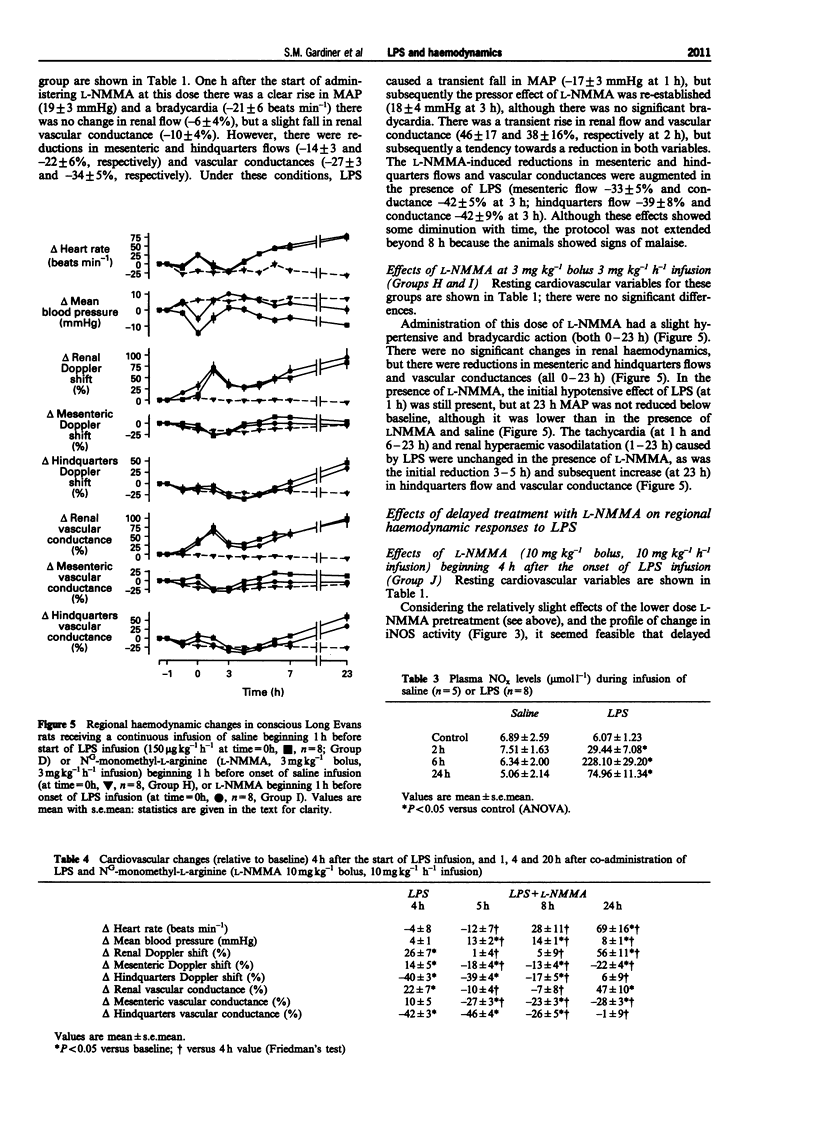
1. A reproducible model of the hyperdynamic circulatory sequelae of endotoxaemia in conscious, chronically-instrumented Long Evans rats, was achieved with a continuous infusion of lipopolysaccharide (LPS, 150 micro g kg(-1) h(-1)) for 32 h. Over the first 2 h of LPS infusion, there was a transient hypotension and tachycardia, accompanied by a marked increase in renal flow and vascular conductance, although there were reductions in cardiac and stroke index. Between 4-8 after the start of LPS infusion, there was slight hypotension and tachycardia, and a transient rise in mesenteric flow and conductance, but reductions in the hindquarters vascular bed; the hyperaemic vasodilatation in the renal vascular bed was maintained. At this stage, all cardiac haemodynamic variables were not different from baseline. At this stage, cardiac and stroke index were substantially elevated, in association with marked increases in peak aortic flow, dF/dtmax and total peripheral conductance; these changes were well-maintained over the following 8 h of LPS infusion. 2. By 2 h after the start of LPS infusion, only lung inducible nitric oxide synthase (iNOS) activity was increased, but at 6 h there were significant increases in iNOS activity in lung, liver, spleen, heart and aorta. (43.3 +/- 7.8, 28.8 +/- 3.3, 50.8 +/- 7.2, 3.04 +/- 0.29, 3.76 +/- 0.94 pmol min(-1) mg(-1) protein, respectively). However, by 24 h after the start of LPS infusion, iNOS activity was not elevated significantly in any tissue examined, and kidney iNOS activity did not change significantly during LPS infusion. Plasma nitrite/nitrate levels were increased after 2 h infusion of LPS (from 6.07 +/- 1.23 to 29.44 +/- 7.08 micromol l(-1)), and further by 6 h (228.10 +/- 29.20 micromol l(-1)), but were less 24 h after onset of LPS infusion (74.96 +/- 11.34 micromol l(-1)). Hence, the progressive hypotension, increasing cardiac function and developing hyperaemic vasodilatation in renal and hindquarters vascular beds between 8-24 h after the onset of LPS infusion, occurred when tissue iNOS activity and plasma nitrite/nitrate levels were falling. 3. Pretreatment with NG-monomethyl-L-arginine (L-NMMA, 30 mg kg(-1) bolus, 30 mg kg(-1) h(-1) infusion) 1 h before LPS infusion did not prevent the early hypotension, but abolished the initial renal vasodilatation and the later (6-8 h) fall in mean arterial pressure (MAP), and the additional renal vasodilatation.
Full text
PDF


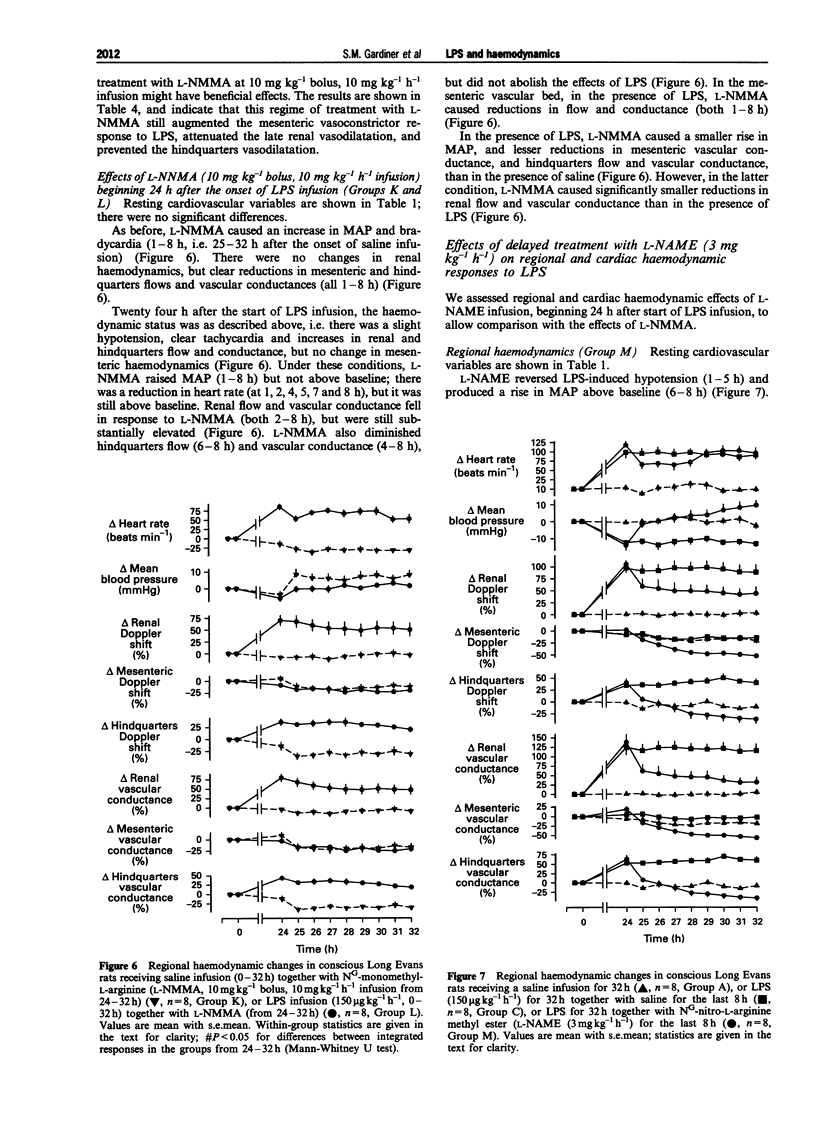
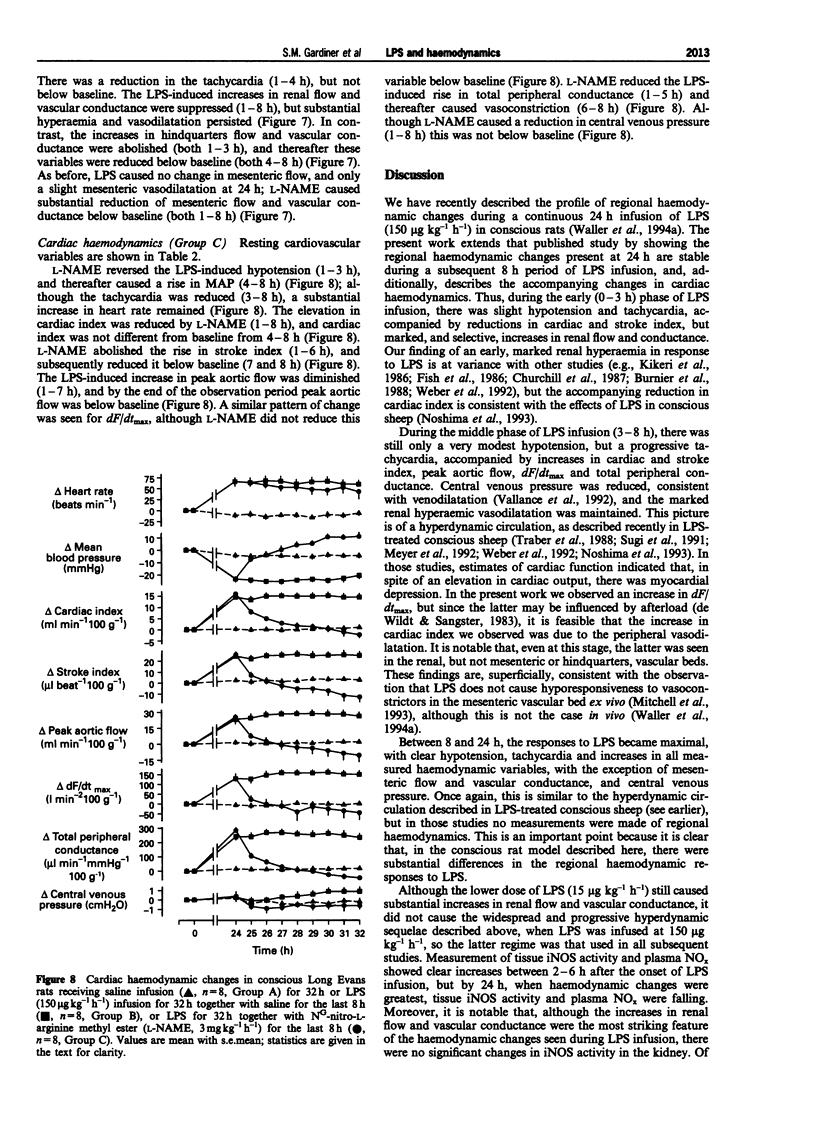



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bone R. C. Why new definitions of sepsis and organ failure are needed. Am J Med. 1993 Oct;95(4):348–350. doi: 10.1016/0002-9343(93)90301-5. [DOI] [PubMed] [Google Scholar]
- Brackett D. J., Schaefer C. F., Tompkins P., Fagraeus L., Peters L. J., Wilson M. F. Evaluation of cardiac output, total peripheral vascular resistance, and plasma concentrations of vasopressin in the conscious, unrestrained rat during endotoxemia. Circ Shock. 1985;17(4):273–284. [PubMed] [Google Scholar]
- Burnier M., Waeber B., Aubert J. F., Nussberger J., Brunner H. R. Effects of nonhypotensive endotoxemia in conscious rats: role of prostaglandins. Am J Physiol. 1988 Mar;254(3 Pt 2):H509–H516. doi: 10.1152/ajpheart.1988.254.3.H509. [DOI] [PubMed] [Google Scholar]
- Buxton I. L., Cheek D. J., Eckman D., Westfall D. P., Sanders K. M., Keef K. D. NG-nitro L-arginine methyl ester and other alkyl esters of arginine are muscarinic receptor antagonists. Circ Res. 1993 Feb;72(2):387–395. doi: 10.1161/01.res.72.2.387. [DOI] [PubMed] [Google Scholar]
- Churchill P. C., Bidani A. K., Schwartz M. M. Renal effects of endotoxin in the male rat. Am J Physiol. 1987 Aug;253(2 Pt 2):F244–F250. doi: 10.1152/ajprenal.1987.253.2.F244. [DOI] [PubMed] [Google Scholar]
- Cobb J. P., Natanson C., Hoffman W. D., Lodato R. F., Banks S., Koev C. A., Solomon M. A., Elin R. J., Hosseini J. M., Danner R. L. N omega-amino-L-arginine, an inhibitor of nitric oxide synthase, raises vascular resistance but increases mortality rates in awake canines challenged with endotoxin. J Exp Med. 1992 Oct 1;176(4):1175–1182. doi: 10.1084/jem.176.4.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobb J. P., Natanson C., Quezado Z. M., Hoffman W. D., Koev C. A., Banks S., Correa R., Levi R., Elin R. J., Hosseini J. M. Differential hemodynamic effects of L-NMMA in endotoxemic and normal dogs. Am J Physiol. 1995 Apr;268(4 Pt 2):H1634–H1642. doi: 10.1152/ajpheart.1995.268.4.H1634. [DOI] [PubMed] [Google Scholar]
- Fish R. E., Lang C. H., Spitzer J. A. Regional blood flow during continuous low-dose endotoxin infusion. Circ Shock. 1986;18(4):267–275. [PubMed] [Google Scholar]
- Gardiner S. M., Compton A. M., Kemp P. A., Bennett T. Effects of NG-nitro-L-arginine methyl ester or indomethacin on differential regional and cardiac haemodynamic actions of arginine vasopressin and lysine vasopressin in conscious rats. Br J Pharmacol. 1991 Jan;102(1):65–72. doi: 10.1111/j.1476-5381.1991.tb12133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardiner S. M., Kemp P. A., Bennett T., Palmer R. M., Moncada S. Regional and cardiac haemodynamic effects of NG, NG,dimethyl-L-arginine and their reversibility by vasodilators in conscious rats. Br J Pharmacol. 1993 Dec;110(4):1457–1464. doi: 10.1111/j.1476-5381.1993.tb13985.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardiner S. M., Kemp P. A., Fallgren B., Bennett T. Effects of chronic infusions of alpha-trinositol on regional and cardiac haemodynamics in conscious rats. Br J Pharmacol. 1994 Sep;113(1):129–136. doi: 10.1111/j.1476-5381.1994.tb16184.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray G. A., Schott C., Julou-Schaeffer G., Fleming I., Parratt J. R., Stoclet J. C. The effect of inhibitors of the L-arginine/nitric oxide pathway on endotoxin-induced loss of vascular responsiveness in anaesthetized rats. Br J Pharmacol. 1991 May;103(1):1218–1224. doi: 10.1111/j.1476-5381.1991.tb12327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guc M. O., Furman B. L., Parratt J. R. Endotoxin-induced impairment of vasopressor and vasodepressor responses in the pithed rat. Br J Pharmacol. 1990 Dec;101(4):913–919. doi: 10.1111/j.1476-5381.1990.tb14180.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillou P. J. Biological variation in the development of sepsis after surgery or trauma. Lancet. 1993 Jul 24;342(8865):217–220. doi: 10.1016/0140-6736(93)92303-b. [DOI] [PubMed] [Google Scholar]
- Hecker M., Mitchell J. A., Harris H. J., Katsura M., Thiemermann C., Vane J. R. Endothelial cells metabolize NG-monomethyl-L-arginine to L-citrulline and subsequently to L-arginine. Biochem Biophys Res Commun. 1990 Mar 30;167(3):1037–1043. doi: 10.1016/0006-291x(90)90627-y. [DOI] [PubMed] [Google Scholar]
- Julou-Schaeffer G., Gray G. A., Fleming I., Schott C., Parratt J. R., Stoclet J. C. Loss of vascular responsiveness induced by endotoxin involves L-arginine pathway. Am J Physiol. 1990 Oct;259(4 Pt 2):H1038–H1043. doi: 10.1152/ajpheart.1990.259.4.H1038. [DOI] [PubMed] [Google Scholar]
- Kikeri D., Pennell J. P., Hwang K. H., Jacob A. I., Richman A. V., Bourgoignie J. J. Endotoxemic acute renal failure in awake rats. Am J Physiol. 1986 Jun;250(6 Pt 2):F1098–F1106. doi: 10.1152/ajprenal.1986.250.6.F1098. [DOI] [PubMed] [Google Scholar]
- Kilbourn R. G., Jubran A., Gross S. S., Griffith O. W., Levi R., Adams J., Lodato R. F. Reversal of endotoxin-mediated shock by NG-methyl-L-arginine, an inhibitor of nitric oxide synthesis. Biochem Biophys Res Commun. 1990 Nov 15;172(3):1132–1138. doi: 10.1016/0006-291x(90)91565-a. [DOI] [PubMed] [Google Scholar]
- Klabunde R. E., Helgren M. C. Cardiovascular actions of NG-methyl-L-arginine are abolished in a canine shock model using high-dose endotoxin. Res Commun Chem Pathol Pharmacol. 1992 Oct;78(1):57–68. [PubMed] [Google Scholar]
- Klabunde R. E., Ritger R. C. NG-monomethyl-l-arginine (NMA) restores arterial blood pressure but reduces cardiac output in a canine model of endotoxic shock. Biochem Biophys Res Commun. 1991 Aug 15;178(3):1135–1140. doi: 10.1016/0006-291x(91)91010-a. [DOI] [PubMed] [Google Scholar]
- Lorente J. A., Landín L., De Pablo R., Renes E., Liste D. L-arginine pathway in the sepsis syndrome. Crit Care Med. 1993 Sep;21(9):1287–1295. doi: 10.1097/00003246-199309000-00010. [DOI] [PubMed] [Google Scholar]
- Meyer J., Traber L. D., Nelson S., Lentz C. W., Nakazawa H., Herndon D. N., Noda H., Traber D. L. Reversal of hyperdynamic response to continuous endotoxin administration by inhibition of NO synthesis. J Appl Physiol (1985) 1992 Jul;73(1):324–328. doi: 10.1152/jappl.1992.73.1.324. [DOI] [PubMed] [Google Scholar]
- Mitchell J. A., Kohlhaas K. L., Sorrentino R., Warner T. D., Murad F., Vane J. R. Induction by endotoxin of nitric oxide synthase in the rat mesentery: lack of effect on action of vasoconstrictors. Br J Pharmacol. 1993 May;109(1):265–270. doi: 10.1111/j.1476-5381.1993.tb13563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noshima S., Noda H., Herndon D. N., Traber L. D., Traber D. L. Left ventricular performance during continuous endotoxin-induced hyperdynamic endotoxemia in sheep. J Appl Physiol (1985) 1993 Apr;74(4):1528–1533. doi: 10.1152/jappl.1993.74.4.1528. [DOI] [PubMed] [Google Scholar]
- Parrillo J. E. Pathogenetic mechanisms of septic shock. N Engl J Med. 1993 May 20;328(20):1471–1477. doi: 10.1056/NEJM199305203282008. [DOI] [PubMed] [Google Scholar]
- Paya D., Gray G. A., Fleming I., Stoclet J. C. Effect of dexamethasone on the onset and persistence of vascular hyporeactivity induced by E. coli lipopolysaccharide in rats. Circ Shock. 1993 Oct;41(2):103–112. [PubMed] [Google Scholar]
- Petros A., Bennett D., Vallance P. Effect of nitric oxide synthase inhibitors on hypotension in patients with septic shock. Lancet. 1991 Dec 21;338(8782-8783):1557–1558. doi: 10.1016/0140-6736(91)92376-d. [DOI] [PubMed] [Google Scholar]
- Petros A., Lamb G., Leone A., Moncada S., Bennett D., Vallance P. Effects of a nitric oxide synthase inhibitor in humans with septic shock. Cardiovasc Res. 1994 Jan;28(1):34–39. doi: 10.1093/cvr/28.1.34. [DOI] [PubMed] [Google Scholar]
- Redl H., Bahrami S., Schlag G., Traber D. L. Clinical detection of LPS and animal models of endotoxemia. Immunobiology. 1993 Apr;187(3-5):330–345. doi: 10.1016/S0171-2985(11)80348-7. [DOI] [PubMed] [Google Scholar]
- Rees D. D., Cellek S., Palmer R. M., Moncada S. Dexamethasone prevents the induction by endotoxin of a nitric oxide synthase and the associated effects on vascular tone: an insight into endotoxin shock. Biochem Biophys Res Commun. 1990 Dec 14;173(2):541–547. doi: 10.1016/s0006-291x(05)80068-3. [DOI] [PubMed] [Google Scholar]
- Rees D. D., Cunha F. Q., Assreuy J., Herman A. G., Moncada S. Sequential induction of nitric oxide synthase by Corynebacterium parvum in different organs of the mouse. Br J Pharmacol. 1995 Feb;114(3):689–693. doi: 10.1111/j.1476-5381.1995.tb17193.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller M. D., Waeber B., Nussberger J., Brunner H. R. Angiotensin II, vasopressin, and sympathetic activity in conscious rats with endotoxemia. Am J Physiol. 1985 Dec;249(6 Pt 2):H1086–H1092. doi: 10.1152/ajpheart.1985.249.6.H1086. [DOI] [PubMed] [Google Scholar]
- Schott C. A., Gray G. A., Stoclet J. C. Dependence of endotoxin-induced vascular hyporeactivity on extracellular L-arginine. Br J Pharmacol. 1993 Jan;108(1):38–43. doi: 10.1111/j.1476-5381.1993.tb13436.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz R., Panas D. L., Catena R., Moncada S., Olley P. M., Lopaschuk G. D. The role of nitric oxide in cardiac depression induced by interleukin-1 beta and tumour necrosis factor-alpha. Br J Pharmacol. 1995 Jan;114(1):27–34. doi: 10.1111/j.1476-5381.1995.tb14901.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suffredini A. F., Fromm R. E., Parker M. M., Brenner M., Kovacs J. A., Wesley R. A., Parrillo J. E. The cardiovascular response of normal humans to the administration of endotoxin. N Engl J Med. 1989 Aug 3;321(5):280–287. doi: 10.1056/NEJM198908033210503. [DOI] [PubMed] [Google Scholar]
- Sugi K., Newald J., Traber L. D., Maguire J. P., Herndon D. N., Schlag G., Traber D. L. Cardiac dysfunction after acute endotoxin administration in conscious sheep. Am J Physiol. 1991 May;260(5 Pt 2):H1474–H1481. doi: 10.1152/ajpheart.1991.260.5.H1474. [DOI] [PubMed] [Google Scholar]
- Sugiura M., Inagami T., Kon V. Endotoxin stimulates endothelin-release in vivo and in vitro as determined by radioimmunoassay. Biochem Biophys Res Commun. 1989 Jun 30;161(3):1220–1227. doi: 10.1016/0006-291x(89)91372-7. [DOI] [PubMed] [Google Scholar]
- Szabó C., Mitchell J. A., Thiemermann C., Vane J. R. Nitric oxide-mediated hyporeactivity to noradrenaline precedes the induction of nitric oxide synthase in endotoxin shock. Br J Pharmacol. 1993 Mar;108(3):786–792. doi: 10.1111/j.1476-5381.1993.tb12879.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiemermann C., Vane J. Inhibition of nitric oxide synthesis reduces the hypotension induced by bacterial lipopolysaccharides in the rat in vivo. Eur J Pharmacol. 1990 Jul 17;182(3):591–595. doi: 10.1016/0014-2999(90)90062-b. [DOI] [PubMed] [Google Scholar]
- Traber D. L., Redl H., Schlag G., Herndon D. N., Kimura R., Prien T., Traber L. D. Cardiopulmonary responses to continuous administration of endotoxin. Am J Physiol. 1988 May;254(5 Pt 2):H833–H839. doi: 10.1152/ajpheart.1988.254.5.H833. [DOI] [PubMed] [Google Scholar]
- Vallance P., Palmer R. M., Moncada S. The role of induction of nitric oxide synthesis in the altered responses of jugular veins from endotoxaemic rabbits. Br J Pharmacol. 1992 Jun;106(2):459–463. doi: 10.1111/j.1476-5381.1992.tb14356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waller J., Gardiner S. M., Bennett T. Regional haemodynamic responses to acetylcholine, methoxamine, salbutamol and bradykinin during lipopolysaccharide infusion in conscious rats. Br J Pharmacol. 1994 Aug;112(4):1057–1064. doi: 10.1111/j.1476-5381.1994.tb13190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber A., Schwieger I. M., Poinsot O., Klohn M., Gaumann D. M., Morel D. R. Sequential changes in renal oxygen consumption and sodium transport during hyperdynamic sepsis in sheep. Am J Physiol. 1992 Jun;262(6 Pt 2):F965–F971. doi: 10.1152/ajprenal.1992.262.6.F965. [DOI] [PubMed] [Google Scholar]
- Wright C. E., Rees D. D., Moncada S. Protective and pathological roles of nitric oxide in endotoxin shock. Cardiovasc Res. 1992 Jan;26(1):48–57. doi: 10.1093/cvr/26.1.48. [DOI] [PubMed] [Google Scholar]
- de Wildt D. J., Sangster B. An evaluation of derived aortic flow parameters as indices of myocardial contractility in rats. J Pharmacol Methods. 1983 Aug;10(1):55–64. doi: 10.1016/0160-5402(83)90014-1. [DOI] [PubMed] [Google Scholar]


