Abstract
1. The present study examined whether cholinoceptor stimulation modulates the release of arachidonic acid-derived mediators from rat isolate tracheae. 2. Tracheae were preincubated with [3H]-arachidonic acid and the outflow of 3H-compounds was determined. Acetylcholine and the muscarinic agonist, carbachol but not nicotine, increased the rate of tritium outflow maximally by about 30%. The M3 receptor-preferring antagonist rho-fluoro-hexahydrosiladiphenidol was more effective than pirenzepine and methoctramine in antagonizing the effect of acetylcholine. 3. High performance liquid chromatography analysis (methanol gradient) of the released 3H-compounds showed that one peak, co-eluting with [14C]-prostaglandin E2([14C]-PGE2) and [3H]-PGD2, was enhanced almost 10 fold following muscarinic receptor activation, whereas the outflow of [3H]-arachidonic acid remained unaffected. 4. Using an acetonitril gradient separation it was shown that [3H]-PGE2, [3H]-PGD2 and [3H]-PGF2alpha are released spontaneously, but [3H]-PGE2 represented the major fraction of 3H-prostaglandins. Acetylcholine enhanced the release of all three 3H-prostaglandins, but the effect on PGE2 was most pronounced and most consistent. 5. After removal of the mucosa the muscarinic effect of acetylcholine on total tritium and on that of the 3H-prostaglandins ([3H]-PGE2/PGD2 peak) was abolished. 6. Acetylcholine also enhanced the outflow of radioimmunologically determined PGE2 in a mucosa-dependent manner. 7. After inhibition of cyclo-oxygenase by 3 microM indomethacin, the outflow of 3H-prostaglandins was reduced to almost undetectable levels and acetylcholine evoked a marked release [3H]-arachidonic acid. The phospholipase A2 inhibitor, quinacrine (up to 100 microM) also blocked the effect of acetylcholine on the outflow of 3H-prostaglandins, but this was not followed by a compensatory increase in the outflow of [3H]-arachidonic acid. 8. In conclusion, activation of muscarinic receptors which have characteristics of the M3 subtype can evoke release of prostaglandins from the airway mucosa.
Full text
PDF

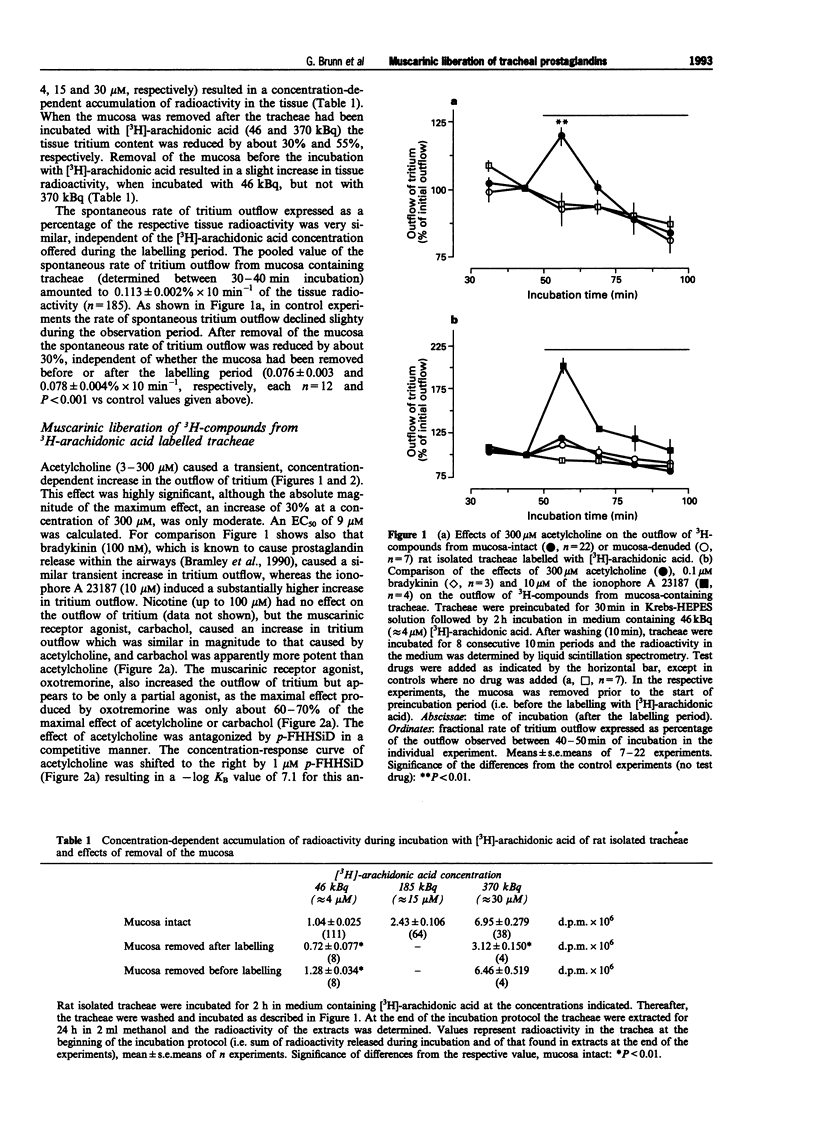
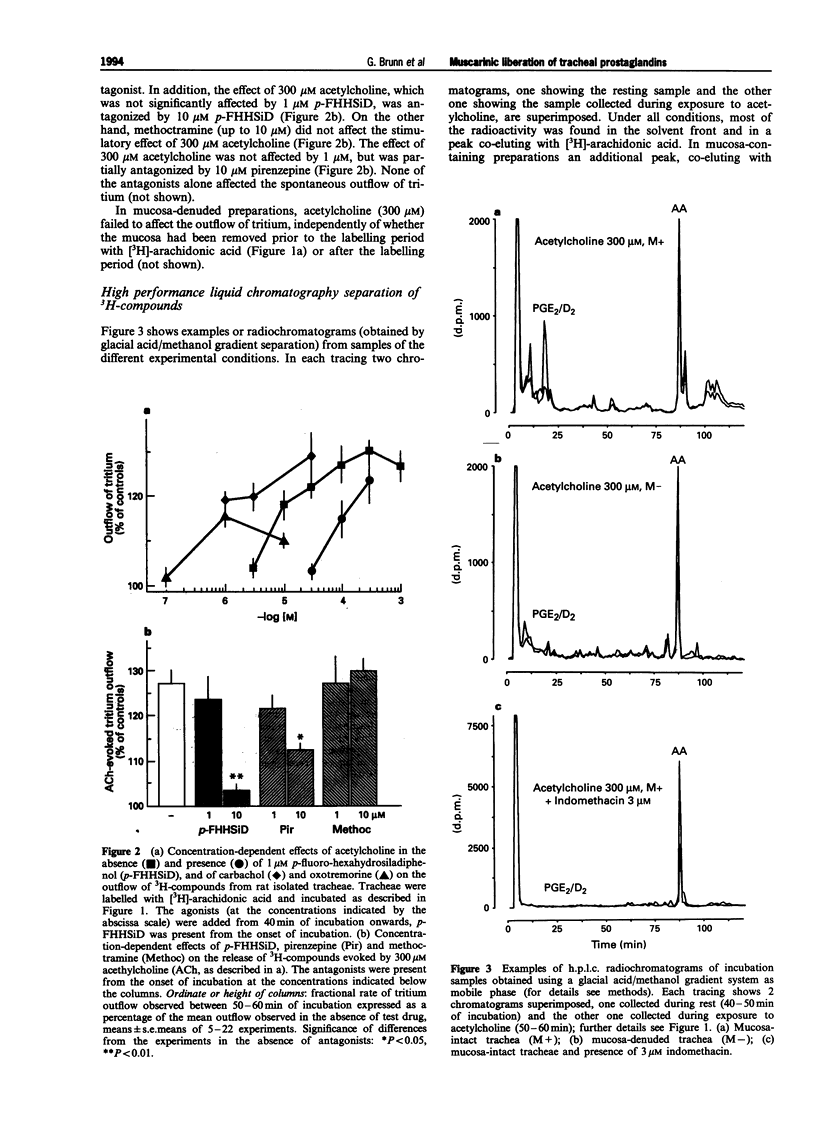
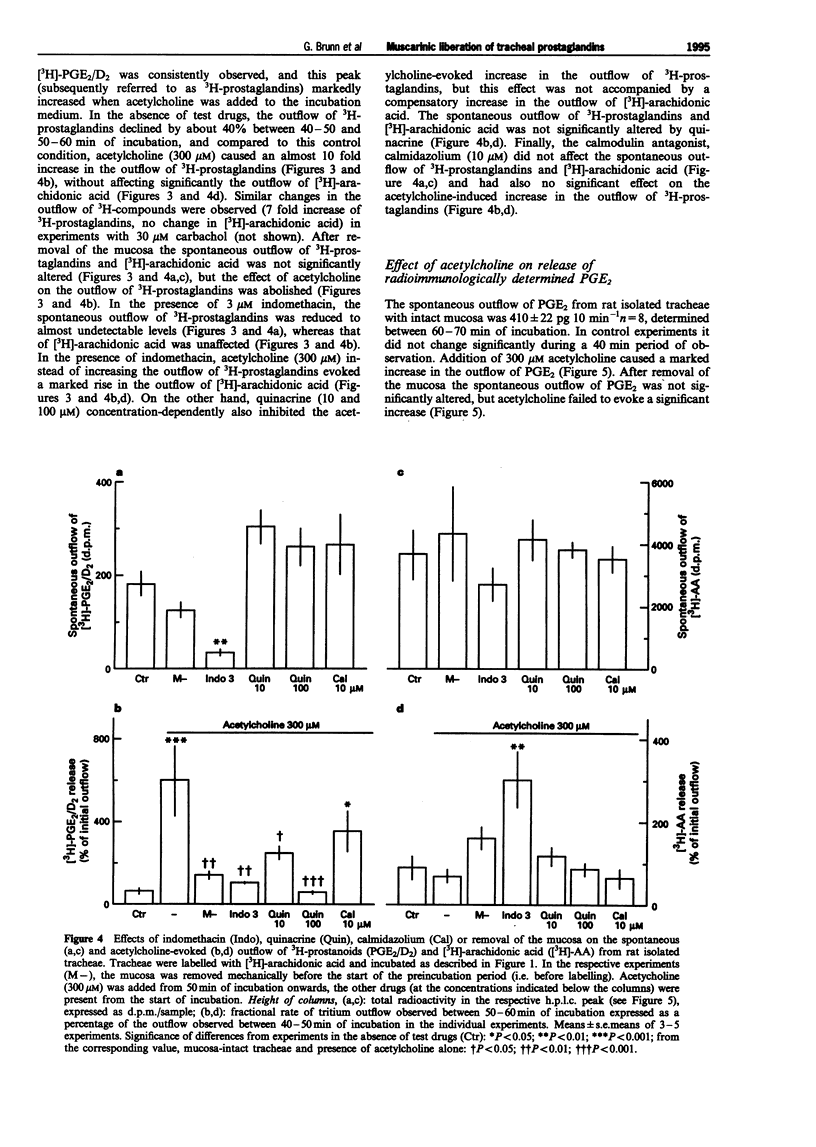
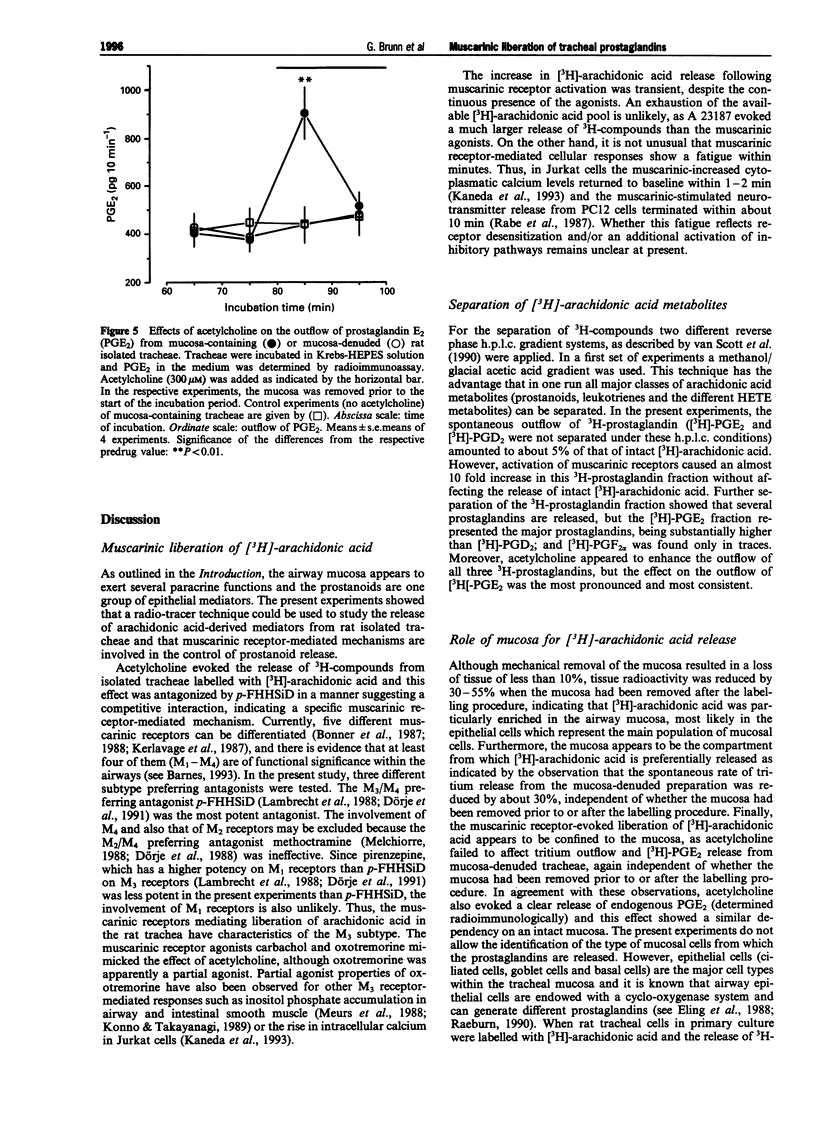


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axelrod J., Burch R. M., Jelsema C. L. Receptor-mediated activation of phospholipase A2 via GTP-binding proteins: arachidonic acid and its metabolites as second messengers. Trends Neurosci. 1988 Mar;11(3):117–123. doi: 10.1016/0166-2236(88)90157-9. [DOI] [PubMed] [Google Scholar]
- Axelrod J. Receptor-mediated activation of phospholipase A2 and arachidonic acid release in signal transduction. Biochem Soc Trans. 1990 Aug;18(4):503–507. doi: 10.1042/bst0180503. [DOI] [PubMed] [Google Scholar]
- Barnes P. J. Muscarinic receptor subtypes in airways. Life Sci. 1993;52(5-6):521–527. doi: 10.1016/0024-3205(93)90310-y. [DOI] [PubMed] [Google Scholar]
- Basbaum C. B., Grillo M. A., Widdicombe J. H. Muscarinic receptors: evidence for a nonuniform distribution in tracheal smooth muscle and exocrine glands. J Neurosci. 1984 Feb;4(2):508–520. doi: 10.1523/JNEUROSCI.04-02-00508.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwell G. J., Flower R. J., Nijkamp F. P., Vane J. R. Phospholipase A2 activity of guinea-pig isolated perfused lungs: stimulation, and inhibition by anti-inflammatory steroids. Br J Pharmacol. 1978 Jan;62(1):79–89. doi: 10.1111/j.1476-5381.1978.tb07009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner T. I., Buckley N. J., Young A. C., Brann M. R. Identification of a family of muscarinic acetylcholine receptor genes. Science. 1987 Jul 31;237(4814):527–532. doi: 10.1126/science.3037705. [DOI] [PubMed] [Google Scholar]
- Bonner T. I., Young A. C., Brann M. R., Buckley N. J. Cloning and expression of the human and rat m5 muscarinic acetylcholine receptor genes. Neuron. 1988 Jul;1(5):403–410. doi: 10.1016/0896-6273(88)90190-0. [DOI] [PubMed] [Google Scholar]
- Boushey H. A., Holtzman M. J. Experimental airway inflammation and hyperreactivity. Searching for cells and mediators. Am Rev Respir Dis. 1985 Mar;131(3):312–313. doi: 10.1164/arrd.1985.131.3.312. [DOI] [PubMed] [Google Scholar]
- Bramley A. M., Samhoun M. N., Piper P. J. The role of the epithelium in modulating the responses of guinea-pig trachea induced by bradykinin in vitro. Br J Pharmacol. 1990 Apr;99(4):762–766. doi: 10.1111/j.1476-5381.1990.tb13003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conklin B. R., Brann M. R., Buckley N. J., Ma A. L., Bonner T. I., Axelrod J. Stimulation of arachidonic acid release and inhibition of mitogenesis by cloned genes for muscarinic receptor subtypes stably expressed in A9 L cells. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8698–8702. doi: 10.1073/pnas.85.22.8698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUNNILL M. S. The pathology of asthma, with special reference to changes in the bronchial mucosa. J Clin Pathol. 1960 Jan;13:27–33. doi: 10.1136/jcp.13.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deckers I. A., Rampart M., Bult H., Herman A. G. Evidence for the involvement of prostaglandins in modulation of acetylcholine release from canine bronchial tissue. Eur J Pharmacol. 1989 Aug 29;167(3):415–418. doi: 10.1016/0014-2999(89)90451-2. [DOI] [PubMed] [Google Scholar]
- Dörje F., Wess J., Lambrecht G., Tacke R., Mutschler E., Brann M. R. Antagonist binding profiles of five cloned human muscarinic receptor subtypes. J Pharmacol Exp Ther. 1991 Feb;256(2):727–733. [PubMed] [Google Scholar]
- Eling T. E., Henke D., Danilowicz R. Arachidonic acid metabolism in respiratory epithelial cells. Gen Pharmacol. 1988;19(3):313–316. doi: 10.1016/0306-3623(88)90022-5. [DOI] [PubMed] [Google Scholar]
- Farmer S. G., Hay D. W., Raeburn D., Fedan J. S. Relaxation of guinea-pig tracheal smooth muscle to arachidonate is converted to contraction following epithelium removal. Br J Pharmacol. 1987 Sep;92(1):231–236. doi: 10.1111/j.1476-5381.1987.tb11316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldie R. G., Fernandes L. B., Farmer S. G., Hay D. W. Airway epithelium-derived inhibitory factor. Trends Pharmacol Sci. 1990 Feb;11(2):67–70. doi: 10.1016/0165-6147(90)90320-8. [DOI] [PubMed] [Google Scholar]
- Henke D. C., Kouzan S., Eling T. E. Analysis of leukotrienes, prostaglandins, and other oxygenated metabolites of arachidonic acid by high-performance liquid chromatography. Anal Biochem. 1984 Jul;140(1):87–94. doi: 10.1016/0003-2697(84)90137-4. [DOI] [PubMed] [Google Scholar]
- Kaneda T., Kitamura Y., Nomura Y. Presence of m3 subtype muscarinic acetylcholine receptors and receptor-mediated increases in the cytoplasmic concentration of Ca2+ in Jurkat, a human leukemic helper T lymphocyte line. Mol Pharmacol. 1993 Mar;43(3):356–364. [PubMed] [Google Scholar]
- Konno F., Takayanagi I. Relationship between the contractile responses and their coupling second messenger systems for muscarinic drugs in the guinea-pig ileal longitudinal muscle. Arch Int Pharmacodyn Ther. 1989 Sep-Oct;301:15–29. [PubMed] [Google Scholar]
- Laitinen L. A., Heino M., Laitinen A., Kava T., Haahtela T. Damage of the airway epithelium and bronchial reactivity in patients with asthma. Am Rev Respir Dis. 1985 Apr;131(4):599–606. doi: 10.1164/arrd.1985.131.4.599. [DOI] [PubMed] [Google Scholar]
- Lambrecht G., Feifel R., Forth B., Strohmann C., Tacke R., Mutschler E. p-fluoro-hexahydro-sila-difenidol: the first M2 beta-selective muscarinic antagonist. Eur J Pharmacol. 1988 Jul 26;152(1-2):193–194. doi: 10.1016/0014-2999(88)90856-4. [DOI] [PubMed] [Google Scholar]
- Lamport S. J., Fedan J. S. Modulation of the reactivity of the guinea-pig isolated trachealis by respiratory epithelium: effects of cooling. Br J Pharmacol. 1990 Feb;99(2):369–373. doi: 10.1111/j.1476-5381.1990.tb14710.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madison J. M., Jones C. A., Tom-Moy M., Brown J. K. Affinities of pirenzepine for muscarinic cholinergic receptors in membranes isolated from bovine tracheal mucosa and smooth muscle. Am Rev Respir Dis. 1987 Mar;135(3):719–724. doi: 10.1164/arrd.1987.135.3.719. [DOI] [PubMed] [Google Scholar]
- Melchiorre C. Polymethylene tetramines: a new generation of selective muscarinic antagonists. Trends Pharmacol Sci. 1988 Jun;9(6):216–220. doi: 10.1016/0165-6147(88)90089-2. [DOI] [PubMed] [Google Scholar]
- Meurs H., Roffel A. F., Postema J. B., Timmermans A., Elzinga C. R., Kauffman H. F., Zaagsma J. Evidence for a direct relationship between phosphoinositide metabolism and airway smooth muscle contraction induced by muscarinic agonists. Eur J Pharmacol. 1988 Nov 1;156(2):271–274. doi: 10.1016/0014-2999(88)90331-7. [DOI] [PubMed] [Google Scholar]
- Rabe C. S., Delorme E., Weight F. F. Muscarine-stimulated neurotransmitter release from PC12 cells. J Pharmacol Exp Ther. 1987 Nov;243(2):534–541. [PubMed] [Google Scholar]
- Racké K., Brunn G., Elsner M., Wessler I. Effects of indomethacin on muscarinic inhibition of endogenous noradrenaline release from rat isolated trachea. Naunyn Schmiedebergs Arch Pharmacol. 1993 Jul;348(1):21–27. doi: 10.1007/BF00168532. [DOI] [PubMed] [Google Scholar]
- Racké K., Bähring A., Brunn G., Elsner M., Wessler I. Characterization of endogenous noradrenaline release from intact and epithelium-denuded rat isolated trachea. Br J Pharmacol. 1991 May;103(1):1213–1217. doi: 10.1111/j.1476-5381.1991.tb12326.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racké K., Bähring J., Langer C., Bräutigam M., Wessler I. Prostanoids inhibit release of endogenous norepinephrine from rat isolated trachea. Am Rev Respir Dis. 1992 Nov;146(5 Pt 1):1182–1186. doi: 10.1164/ajrccm/146.5_Pt_1.1182. [DOI] [PubMed] [Google Scholar]
- Raeburn D. Eicosanoids, epithelium and airway reactivity. Gen Pharmacol. 1990;21(1):11–16. doi: 10.1016/0306-3623(90)90587-c. [DOI] [PubMed] [Google Scholar]
- Reimann A., Brunn G., Hey C., Wessler I., Racké K. Arachidonic acid liberation from rat tracheal epithelial cells by alveolar macrophages. Adv Prostaglandin Thromboxane Leukot Res. 1995;23:357–359. [PubMed] [Google Scholar]
- Tschirhart E., Frossard N., Bertrand C., Landry Y. Arachidonic acid metabolites and airway epithelium-dependent relaxant factor. J Pharmacol Exp Ther. 1987 Oct;243(1):310–316. [PubMed] [Google Scholar]
- Van Scott M. R., McIntire M. R., Henke D. C. Arachidonic acid metabolism and regulation of ion transport in rabbit Clara cells. Am J Physiol. 1990 Oct;259(4 Pt 1):L213–L221. doi: 10.1152/ajplung.1990.259.4.L213. [DOI] [PubMed] [Google Scholar]
- Wallenstein S., Zucker C. L., Fleiss J. L. Some statistical methods useful in circulation research. Circ Res. 1980 Jul;47(1):1–9. doi: 10.1161/01.res.47.1.1. [DOI] [PubMed] [Google Scholar]
- Wessler I., Bender H., Härle P., Höhle K. D., Kirdorf G., Klapproth H., Reinheimer T., Rícný J., Schniepp-Mendelssohn K. E., Racké K. Release of [3H]acetylcholine in human isolated bronchi. Effect of indomethacin on muscarinic autoinhibition. Am J Respir Crit Care Med. 1995 Apr;151(4):1040–1046. doi: 10.1164/ajrccm.151.4.7697228. [DOI] [PubMed] [Google Scholar]
- Wessler I., Hellwig D., Racké K. Epithelium-derived inhibition of [3H]acetylcholine release from the isolated guinea-pig trachea. Naunyn Schmiedebergs Arch Pharmacol. 1990 Oct;342(4):387–393. doi: 10.1007/BF00169454. [DOI] [PubMed] [Google Scholar]
- Wessler I., Reinheimer T., Brunn G., Anderson G. P., Maclagan J., Racké K. Beta-adrenoceptors mediate inhibition of [3H]-acetylcholine release from the isolated rat and guinea-pig trachea: role of the airway mucosa and prostaglandins. Br J Pharmacol. 1994 Dec;113(4):1221–1230. doi: 10.1111/j.1476-5381.1994.tb17128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]



