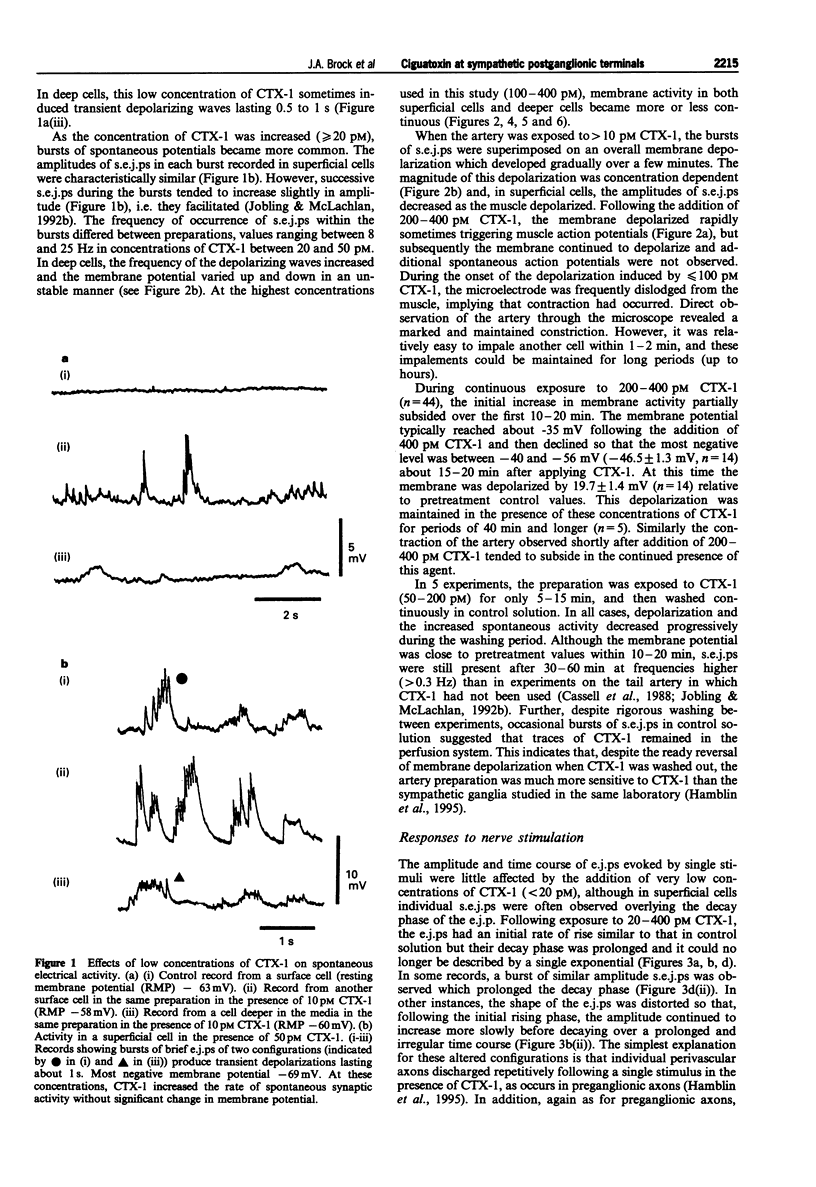
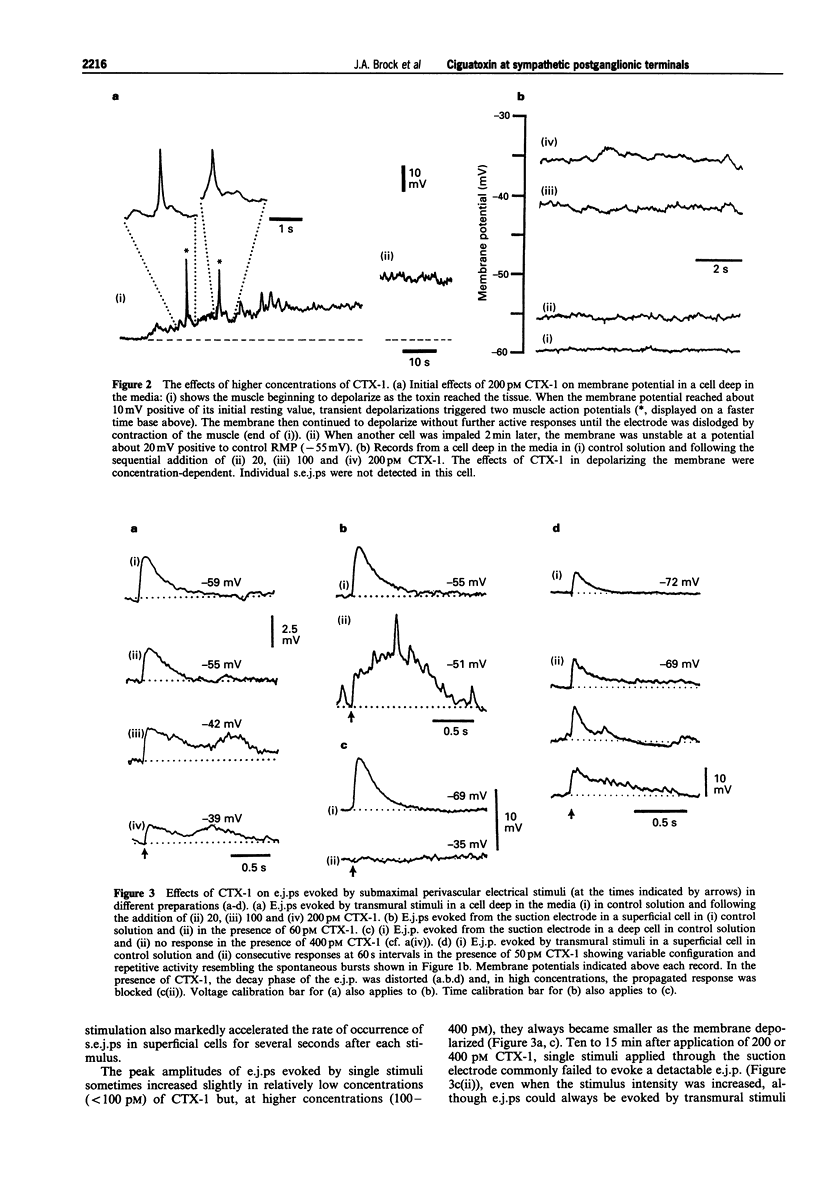
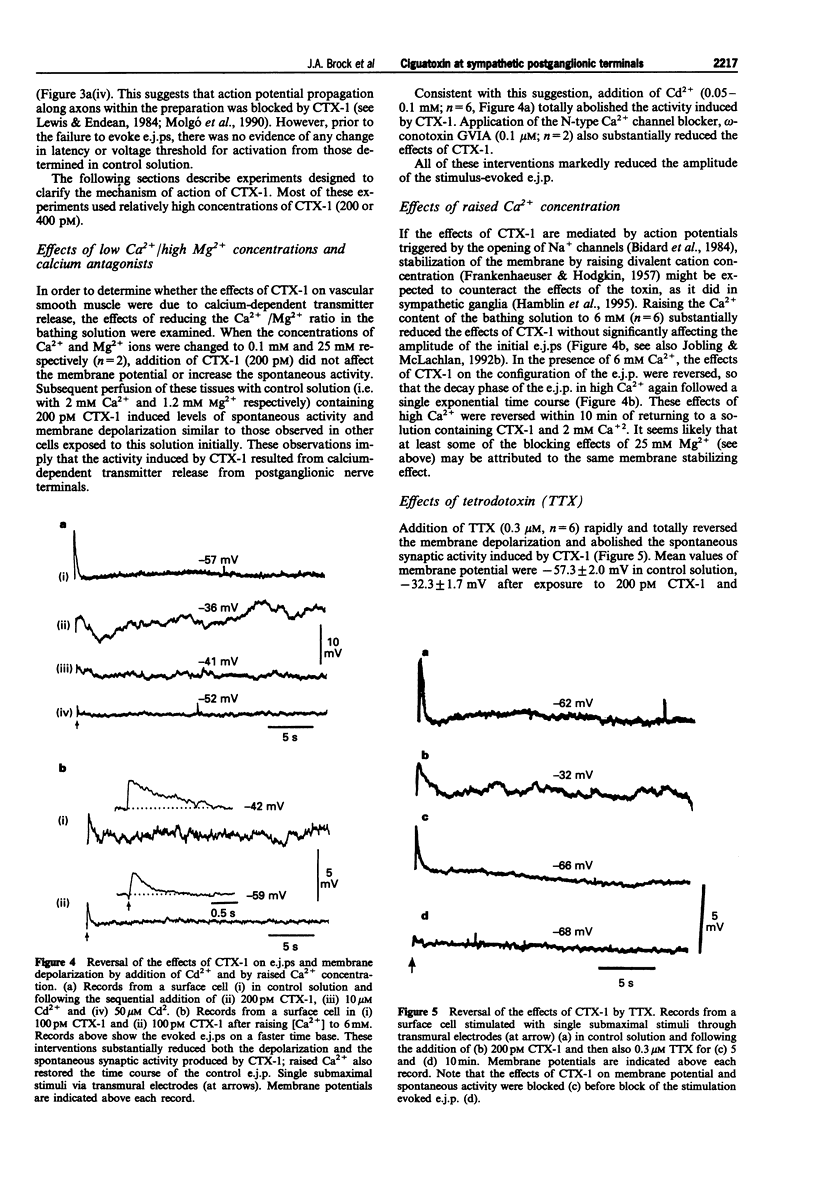
Abstract
1. The effects of ciguatoxin-1 (CTX-1) on the membrane potential of smooth muscle cells have been examined in rat proximal tail arteries isolated in vitro. 2. CTX-1 (> or = 10 pM) increased the frequency of spontaneous excitatory junction potentials (s.e.j.ps). At 100-400 pM, there was also a marked and maintained depolarization (19.7 +/- 1.4 mV, n = 14, at 400 pM). 3. In 20-400 pM CTX-1, perivascular stimuli evoked excitatory junction potentials (e.j.ps) which were prolonged in time course relative to control. 4. Although threshold and latency of the e.j.p. were not affected by CTX-1 (< or = 400 pM), propagated impulses were blocked at > or = 100 pM. 5. The spontaneous activity and the depolarization produced by CTX-1 were reduced in the presence of Ca2+ (0.1 mM)/Mg2+ (25 mM), omega-conotoxin (0.1 microM) or Cd2+ (50-100 microM). 6. All effects of CTX-1 were abolished by tetrodotoxin (0.3 microM). 7. Raised Ca2+ (6 mM) reduced the depolarization and spontaneous activity produced by CTX-1. 8. In 400 pM CTX-1, the membrane repolarized (17 +/- 3.2 mV, n = 4) following the addition of phentolamine (1 microM). S.e.j.ps and e.j.ps were selectively abolished by suramin (1 mM), and the membrane repolarized by 1.3 +/- 1.6 mV (n = 4). 9. We conclude that CTX-1 releases noradrenaline and ATP by initiating asynchronous discharge of postganglionic perivascular axons. In 100-400 pM CTX-1, the smooth muscle was depolarized to levels resembling those recorded in this artery during ongoing vasoconstrictor discharge in vivo.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baden D. G. Brevetoxins: unique polyether dinoflagellate toxins. FASEB J. 1989 May;3(7):1807–1817. doi: 10.1096/fasebj.3.7.2565840. [DOI] [PubMed] [Google Scholar]
- Bao J. X., Eriksson I. E., Stjärne L. Age-related variations in the relative importance of noradrenaline and ATP as mediators of the contractile response of rat tail artery to sympathetic nerve stimulation. Acta Physiol Scand. 1989 Jun;136(2):287–288. doi: 10.1111/j.1748-1716.1989.tb08663.x. [DOI] [PubMed] [Google Scholar]
- Bao J. X., Stjärne L. Dual contractile effects of ATP released by field stimulation revealed by effects of alpha,beta-methylene ATP and suramin in rat tail artery. Br J Pharmacol. 1993 Dec;110(4):1421–1428. doi: 10.1111/j.1476-5381.1993.tb13979.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoit E., Legrand A. M., Dubois J. M. Effects of ciguatoxin on current and voltage clamped frog myelinated nerve fibre. Toxicon. 1986;24(4):357–364. doi: 10.1016/0041-0101(86)90195-9. [DOI] [PubMed] [Google Scholar]
- Bidard J. N., Vijverberg H. P., Frelin C., Chungue E., Legrand A. M., Bagnis R., Lazdunski M. Ciguatoxin is a novel type of Na+ channel toxin. J Biol Chem. 1984 Jul 10;259(13):8353–8357. [PubMed] [Google Scholar]
- Bolton T. B., Large W. A. Are junction potentials essential? Dual mechanism of smooth muscle cell activation by transmitter released from autonomic nerves. Q J Exp Physiol. 1986 Jan;71(1):1–28. doi: 10.1113/expphysiol.1986.sp002960. [DOI] [PubMed] [Google Scholar]
- Brock J. A., Cunnane T. C. Neurotransmitter release mechanisms at the sympathetic neuroeffector junction. Exp Physiol. 1993 Sep;78(5):591–614. doi: 10.1113/expphysiol.1993.sp003709. [DOI] [PubMed] [Google Scholar]
- Bryant H. J., Harder D. R., Pamnani M. B., Haddy F. J. In vivo membrane potentials of smooth muscle cells in the caudal artery of the rat. Am J Physiol. 1985 Jul;249(1 Pt 1):C78–C83. doi: 10.1152/ajpcell.1985.249.1.C78. [DOI] [PubMed] [Google Scholar]
- Cassell J. F., Clark A. L., McLachlan E. M. Characteristics of phasic and tonic sympathetic ganglion cells of the guinea-pig. J Physiol. 1986 Mar;372:457–483. doi: 10.1113/jphysiol.1986.sp016020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassell J. F., McLachlan E. M., Sittiracha T. The effect of temperature on neuromuscular transmission in the main caudal artery of the rat. J Physiol. 1988 Mar;397:31–49. doi: 10.1113/jphysiol.1988.sp016986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall W. A. Cellular and molecular biology of voltage-gated sodium channels. Physiol Rev. 1992 Oct;72(4 Suppl):S15–S48. doi: 10.1152/physrev.1992.72.suppl_4.S15. [DOI] [PubMed] [Google Scholar]
- Cheung D. W. Two components in the cellular response of rat tail arteries to nerve stimulation. J Physiol. 1982 Jul;328:461–468. doi: 10.1113/jphysiol.1982.sp014277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans R. J., Surprenant A. Vasoconstriction of guinea-pig submucosal arterioles following sympathetic nerve stimulation is mediated by the release of ATP. Br J Pharmacol. 1992 Jun;106(2):242–249. doi: 10.1111/j.1476-5381.1992.tb14323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANKENHAEUSER B., HODGKIN A. L. The action of calcium on the electrical properties of squid axons. J Physiol. 1957 Jul 11;137(2):218–244. doi: 10.1113/jphysiol.1957.sp005808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkel A. S., Hirst G. D., Van Helden D. F. Some properties of excitatory junction currents recorded from submucosal arterioles of guinea-pig ileum. J Physiol. 1984 Jun;351:87–98. doi: 10.1113/jphysiol.1984.sp015234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie N. C., Lewis R. J., Pearn J. H., Bourke A. T., Holmes M. J., Bourke J. B., Shields W. J. Ciguatera in Australia. Occurrence, clinical features, pathophysiology and management. Med J Aust. 1986 Dec 1;145(11-12):584–590. [PubMed] [Google Scholar]
- Hirst G. D., Edwards F. R. Sympathetic neuroeffector transmission in arteries and arterioles. Physiol Rev. 1989 Apr;69(2):546–604. doi: 10.1152/physrev.1989.69.2.546. [DOI] [PubMed] [Google Scholar]
- Hirst G. D. Neuromuscular transmission in arterioles of guinea-pig submucosa. J Physiol. 1977 Dec;273(1):263–275. doi: 10.1113/jphysiol.1977.sp012093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh T., Kajikuri J., Kuriyama H. Characteristic features of noradrenaline-induced Ca2+ mobilization and tension in arterial smooth muscle of the rabbit. J Physiol. 1992 Nov;457:297–314. doi: 10.1113/jphysiol.1992.sp019379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh T., Kitamura K., Kuriyama H. Roles of extrajunctional receptors in the response of guinea-pig mesenteric and rat tail arteries to adrenergic nerves. J Physiol. 1983 Dec;345:409–422. doi: 10.1113/jphysiol.1983.sp014985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobling P. Electrophysiological events during neuroeffector transmission in the spleen of guinea-pigs and rats. J Physiol. 1994 Apr 1;476(1):153–165. [PMC free article] [PubMed] [Google Scholar]
- Jobling P., McLachlan E. M. An electrophysiological study of responses evoked in isolated segments of rat tail artery during growth and maturation. J Physiol. 1992 Aug;454:83–105. doi: 10.1113/jphysiol.1992.sp019255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobling P., McLachlan E. M., Jänig W., Anderson C. R. Electrophysiological responses in the rat tail artery during reinnervation following lesions of the sympathetic supply. J Physiol. 1992 Aug;454:107–128. doi: 10.1113/jphysiol.1992.sp019256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jänig W. Pre- and postganglionic vasoconstrictor neurons: differentiation, types, and discharge properties. Annu Rev Physiol. 1988;50:525–539. doi: 10.1146/annurev.ph.50.030188.002521. [DOI] [PubMed] [Google Scholar]
- LILEY A. W. The effects of presynaptic polarization on the spontaneous activity at the mammalian neuromuscular junction. J Physiol. 1956 Nov 28;134(2):427–443. doi: 10.1113/jphysiol.1956.sp005655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis R. J., Endean R. Direct and indirect effects of ciguatoxin on guinea-pig atria and papillary muscles. Naunyn Schmiedebergs Arch Pharmacol. 1986 Nov;334(3):313–322. doi: 10.1007/BF00508787. [DOI] [PubMed] [Google Scholar]
- Lewis R. J., Endean R. Mode of action of ciguatoxin from the Spanish Mackerel, Scomberomorus commersoni, on the guinea-pig ileum and vas deferens. J Pharmacol Exp Ther. 1984 Mar;228(3):756–760. [PubMed] [Google Scholar]
- Lewis R. J., Sellin M., Poli M. A., Norton R. S., MacLeod J. K., Sheil M. M. Purification and characterization of ciguatoxins from moray eel (Lycodontis javanicus, Muraenidae). Toxicon. 1991;29(9):1115–1127. doi: 10.1016/0041-0101(91)90209-a. [DOI] [PubMed] [Google Scholar]
- Lombet A., Bidard J. N., Lazdunski M. Ciguatoxin and brevetoxins share a common receptor site on the neuronal voltage-dependent Na+ channel. FEBS Lett. 1987 Jul 27;219(2):355–359. doi: 10.1016/0014-5793(87)80252-1. [DOI] [PubMed] [Google Scholar]
- Luff S. E., McLachlan E. M. Frequency of neuromuscular junctions on arteries of different dimensions in the rabbit, guinea pig and rat. Blood Vessels. 1989;26(2):95–106. doi: 10.1159/000158758. [DOI] [PubMed] [Google Scholar]
- Molgó J., Comella J. X., Legrand A. M. Ciguatoxin enhances quantal transmitter release from frog motor nerve terminals. Br J Pharmacol. 1990 Apr;99(4):695–700. doi: 10.1111/j.1476-5381.1990.tb12991.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris J. L. Roles of noradrenaline and ATP in sympathetic vasoconstriction of the guinea-pig main ear artery. J Auton Nerv Syst. 1994 Nov;49(3):217–225. doi: 10.1016/0165-1838(94)90168-6. [DOI] [PubMed] [Google Scholar]
- Neild T. O., Keef K. Measurements of the membrane potential of arterial smooth muscle in anesthetized animals and its relationship to changes in artery diameter. Microvasc Res. 1985 Jul;30(1):19–28. doi: 10.1016/0026-2862(85)90034-2. [DOI] [PubMed] [Google Scholar]
- Nilsson H., Jensen P. E., Mulvany M. J. Minor role for direct adrenoceptor-mediated calcium entry in rat mesenteric small arteries. J Vasc Res. 1994 Nov-Dec;31(6):314–321. doi: 10.1159/000159059. [DOI] [PubMed] [Google Scholar]
- Sittiracha T., McLachlan E. M., Bell C. The innervation of the caudal artery of the rat. Neuroscience. 1987 May;21(2):647–659. doi: 10.1016/0306-4522(87)90150-3. [DOI] [PubMed] [Google Scholar]
- Sjöblom-Widfeldt N., Gustafsson H., Nilsson H. Transmitter characteristics of small mesenteric arteries from the rat. Acta Physiol Scand. 1990 Feb;138(2):203–212. doi: 10.1111/j.1748-1716.1990.tb08834.x. [DOI] [PubMed] [Google Scholar]
- Surprenant A. A comparative study of neuromuscular transmission in several mammalian muscular arteries. Pflugers Arch. 1980 Jul;386(1):85–91. doi: 10.1007/BF00584192. [DOI] [PubMed] [Google Scholar]
- Van Helden D. F. Spontaneous and noradrenaline-induced transient depolarizations in the smooth muscle of guinea-pig mesenteric vein. J Physiol. 1991 Jun;437:511–541. doi: 10.1113/jphysiol.1991.sp018609. [DOI] [PMC free article] [PubMed] [Google Scholar]



