Abstract
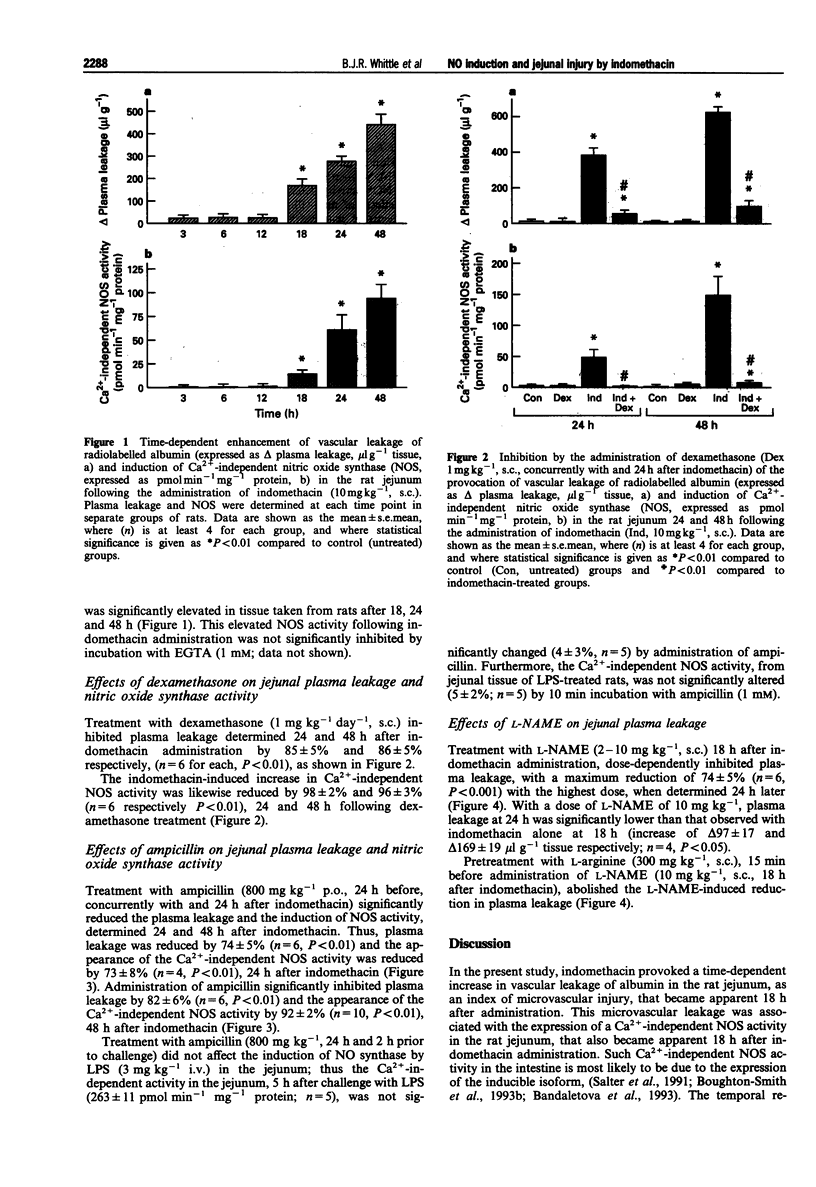
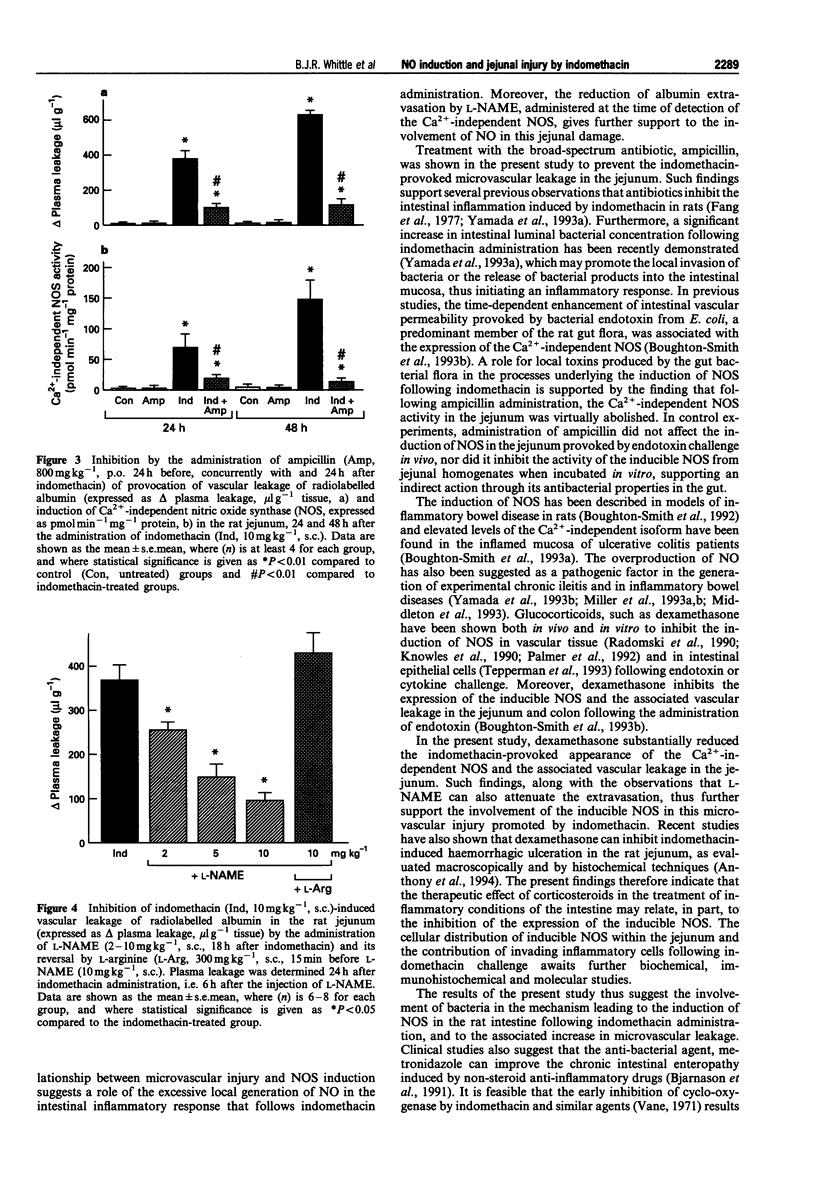
1. The role of nitric oxide (NO) formed by the inducible isoform of NO synthase (NOS) in the generation of indomethacin-induced intestinal microvascular leakage was investigated in the rat. 2. Indomethacin (10 mg kg-1, s.c.) provoked an elevation of vascular leakage of radiolabelled human serum albumin in the jejunum over 48 h, commencing 18 h after its administration. This was associated with the induction of a calcium-independent NOS, as assessed by the conversion of radiolabelled L-arginine to citrulline. 3. Pretreatment with the glucocorticoid, dexamethasone (1 mg kg-1 day-1, s.c.) inhibited the induction of NOS and reduced jejunal microvascular leakage, determined 24 and 48 h after indomethacin. 4. Administration of the broad-spectrum antibiotic, ampicillin (800 mg kg-1 day-1, p.o.) likewise inhibited both the induction of NOS and the plasma leakage observed 24 and 48 h after indomethacin. 5. Ampicillin pretreatment did not, however, inhibit the induction of NOS, determined 5 h following endotoxin (3 mg kg-1 i.v.) challenge. Furthermore, incubation with ampicillin (1 mM, 10 min) did not inhibit the activity of the calcium-independent isoform in vitro. 6. Administration of the NOS inhibitor, NG-nitro-L-arginine methyl ester (L-NAME, 2-10 mg kg-1, s.c.), at the time of the detectable expression of the inducible NOS (18 h after indomethacin), dose-dependently attenuated the plasma leakage, determined 6 later. This effect was reversed by pretreatment with L-arginine (300 mg kg-1, s.c.) 15 min before L-NAME. 7. These findings suggest that induction of a calcium-independent NOS following indomethacin administration involves gut bacteria and leads to microvascular injury in the rat jejunum.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anthony A., Dhillon A. P., Sim R., Pounder R. E., Wakefield A. J. Dexamethasone promotes ulcer plugging in experimental enteritis. Aliment Pharmacol Ther. 1994 Dec;8(6):597–602. doi: 10.1111/j.1365-2036.1994.tb00336.x. [DOI] [PubMed] [Google Scholar]
- Bandaletova T., Brouet I., Bartsch H., Sugimura T., Esumi H., Ohshima H. Immunohistochemical localization of an inducible form of nitric oxide synthase in various organs of rats treated with Propionibacterium acnes and lipopolysaccharide. APMIS. 1993 Apr;101(4):330–336. [PubMed] [Google Scholar]
- Beckman J. S., Beckman T. W., Chen J., Marshall P. A., Freeman B. A. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1620–1624. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjarnason I., Hopkinson N., Zanelli G., Prouse P., Smethurst P., Gumpel J. M., Levi A. J. Treatment of non-steroidal anti-inflammatory drug induced enteropathy. Gut. 1990 Jul;31(7):777–780. doi: 10.1136/gut.31.7.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjarnason I., Zanelli G., Smith T., Prouse P., Williams P., Smethurst P., Delacey G., Gumpel M. J., Levi A. J. Nonsteroidal antiinflammatory drug-induced intestinal inflammation in humans. Gastroenterology. 1987 Sep;93(3):480–489. doi: 10.1016/0016-5085(87)90909-7. [DOI] [PubMed] [Google Scholar]
- Boughton-Smith N. K., Evans S. M., Hawkey C. J., Cole A. T., Balsitis M., Whittle B. J., Moncada S. Nitric oxide synthase activity in ulcerative colitis and Crohn's disease. Lancet. 1993 Aug 7;342(8867):338–340. doi: 10.1016/0140-6736(93)91476-3. [DOI] [PubMed] [Google Scholar]
- Boughton-Smith N. K., Evans S. M., Laszlo F., Whittle B. J., Moncada S. The induction of nitric oxide synthase and intestinal vascular permeability by endotoxin in the rat. Br J Pharmacol. 1993 Nov;110(3):1189–1195. doi: 10.1111/j.1476-5381.1993.tb13940.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang W. F., Broughton A., Jacobson E. D. Indomethacin-induced intestinal inflammation. Am J Dig Dis. 1977 Sep;22(9):749–760. doi: 10.1007/BF01694504. [DOI] [PubMed] [Google Scholar]
- Hogg N., Darley-Usmar V. M., Wilson M. T., Moncada S. Production of hydroxyl radicals from the simultaneous generation of superoxide and nitric oxide. Biochem J. 1992 Jan 15;281(Pt 2):419–424. doi: 10.1042/bj2810419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutcheson I. R., Whittle B. J., Boughton-Smith N. K. Role of nitric oxide in maintaining vascular integrity in endotoxin-induced acute intestinal damage in the rat. Br J Pharmacol. 1990 Dec;101(4):815–820. doi: 10.1111/j.1476-5381.1990.tb14163.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones W. G., 2nd, Barber A. E., Minei J. P., Fahey T. J., Shires G. T., 3rd, Shires G. T. Antibiotic prophylaxis diminishes bacterial translocation but not mortality in experimental burn wound sepsis. J Trauma. 1990 Jun;30(6):737–740. doi: 10.1097/00005373-199006000-00015. [DOI] [PubMed] [Google Scholar]
- Knowles R. G., Salter M., Brooks S. L., Moncada S. Anti-inflammatory glucocorticoids inhibit the induction by endotoxin of nitric oxide synthase in the lung, liver and aorta of the rat. Biochem Biophys Res Commun. 1990 Nov 15;172(3):1042–1048. doi: 10.1016/0006-291x(90)91551-3. [DOI] [PubMed] [Google Scholar]
- Laszlo F., Evans S. M., Whittle B. J. Aminoguanidine inhibits both constitutive and inducible nitric oxide synthase isoforms in rat intestinal microvasculature in vivo. Eur J Pharmacol. 1995 Jan 16;272(2-3):169–175. doi: 10.1016/0014-2999(94)00637-m. [DOI] [PubMed] [Google Scholar]
- Laszlo F., Whittle B. J., Moncada S. Time-dependent enhancement or inhibition of endotoxin-induced vascular injury in rat intestine by nitric oxide synthase inhibitors. Br J Pharmacol. 1994 Apr;111(4):1309–1315. doi: 10.1111/j.1476-5381.1994.tb14887.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton S. J., Shorthouse M., Hunter J. O. Increased nitric oxide synthesis in ulcerative colitis. Lancet. 1993 Feb 20;341(8843):465–466. doi: 10.1016/0140-6736(93)90211-x. [DOI] [PubMed] [Google Scholar]
- Miller M. J., Sadowska-Krowicka H., Chotinaruemol S., Kakkis J. L., Clark D. A. Amelioration of chronic ileitis by nitric oxide synthase inhibition. J Pharmacol Exp Ther. 1993 Jan;264(1):11–16. [PubMed] [Google Scholar]
- Miller M. J., Zhang X. J., Sadowska-Krowicka H., Chotinaruemol S., McIntyre J. A., Clark D. A., Bustamante S. A. Nitric oxide release in response to gut injury. Scand J Gastroenterol. 1993 Feb;28(2):149–154. doi: 10.3109/00365529309096062. [DOI] [PubMed] [Google Scholar]
- Moncada S., Palmer R. M., Higgs E. A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991 Jun;43(2):109–142. [PubMed] [Google Scholar]
- Palmer R. M., Bridge L., Foxwell N. A., Moncada S. The role of nitric oxide in endothelial cell damage and its inhibition by glucocorticoids. Br J Pharmacol. 1992 Jan;105(1):11–12. doi: 10.1111/j.1476-5381.1992.tb14202.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radomski M. W., Palmer R. M., Moncada S. Glucocorticoids inhibit the expression of an inducible, but not the constitutive, nitric oxide synthase in vascular endothelial cells. Proc Natl Acad Sci U S A. 1990 Dec;87(24):10043–10047. doi: 10.1073/pnas.87.24.10043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert A., Asano T. Resistance of germfree rats to indomethacin-induced intestinal lesions. Prostaglandins. 1977 Aug;14(2):333–341. doi: 10.1016/0090-6980(77)90178-2. [DOI] [PubMed] [Google Scholar]
- Salter M., Knowles R. G., Moncada S. Widespread tissue distribution, species distribution and changes in activity of Ca(2+)-dependent and Ca(2+)-independent nitric oxide synthases. FEBS Lett. 1991 Oct 7;291(1):145–149. doi: 10.1016/0014-5793(91)81123-p. [DOI] [PubMed] [Google Scholar]
- Somogyi A., Kovács K., Selye H. Jejunal ulcers produced by indomethacin. J Pharm Pharmacol. 1969 Feb;21(2):122–123. doi: 10.1111/j.2042-7158.1969.tb08211.x. [DOI] [PubMed] [Google Scholar]
- Tepperman B. L., Brown J. F., Whittle B. J. Nitric oxide synthase induction and intestinal epithelial cell viability in rats. Am J Physiol. 1993 Aug;265(2 Pt 1):G214–G218. doi: 10.1152/ajpgi.1993.265.2.G214. [DOI] [PubMed] [Google Scholar]
- Vane J. R. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nat New Biol. 1971 Jun 23;231(25):232–235. doi: 10.1038/newbio231232a0. [DOI] [PubMed] [Google Scholar]
- Wallace J. L., Steel G., Whittle B. J., Lagente V., Vargaftig B. Evidence for platelet-activating factor as a mediator of endotoxin-induced gastrointestinal damage in the rat. Effects of three platelet-activating factor antagonists. Gastroenterology. 1987 Oct;93(4):765–773. doi: 10.1016/0016-5085(87)90438-0. [DOI] [PubMed] [Google Scholar]
- Weissenborn U., Maedge S., Buettner D., Sewing K. F. Indometacin-induced gastrointestinal lesions in relation to tissue concentration, food intake and bacterial invasion in the rat. Pharmacology. 1985;30(1):32–39. doi: 10.1159/000138047. [DOI] [PubMed] [Google Scholar]
- Whittle B. J. Temporal relationship between cyclooxygenase inhibition, as measured by prostacyclin biosynthesis, and the gastrointestinal damage induced by indomethacin in the rat. Gastroenterology. 1981 Jan;80(1):94–98. [PubMed] [Google Scholar]
- Yamada T., Deitch E., Specian R. D., Perry M. A., Sartor R. B., Grisham M. B. Mechanisms of acute and chronic intestinal inflammation induced by indomethacin. Inflammation. 1993 Dec;17(6):641–662. doi: 10.1007/BF00920471. [DOI] [PubMed] [Google Scholar]
- Yamada T., Sartor R. B., Marshall S., Specian R. D., Grisham M. B. Mucosal injury and inflammation in a model of chronic granulomatous colitis in rats. Gastroenterology. 1993 Mar;104(3):759–771. doi: 10.1016/0016-5085(93)91011-6. [DOI] [PubMed] [Google Scholar]