Abstract
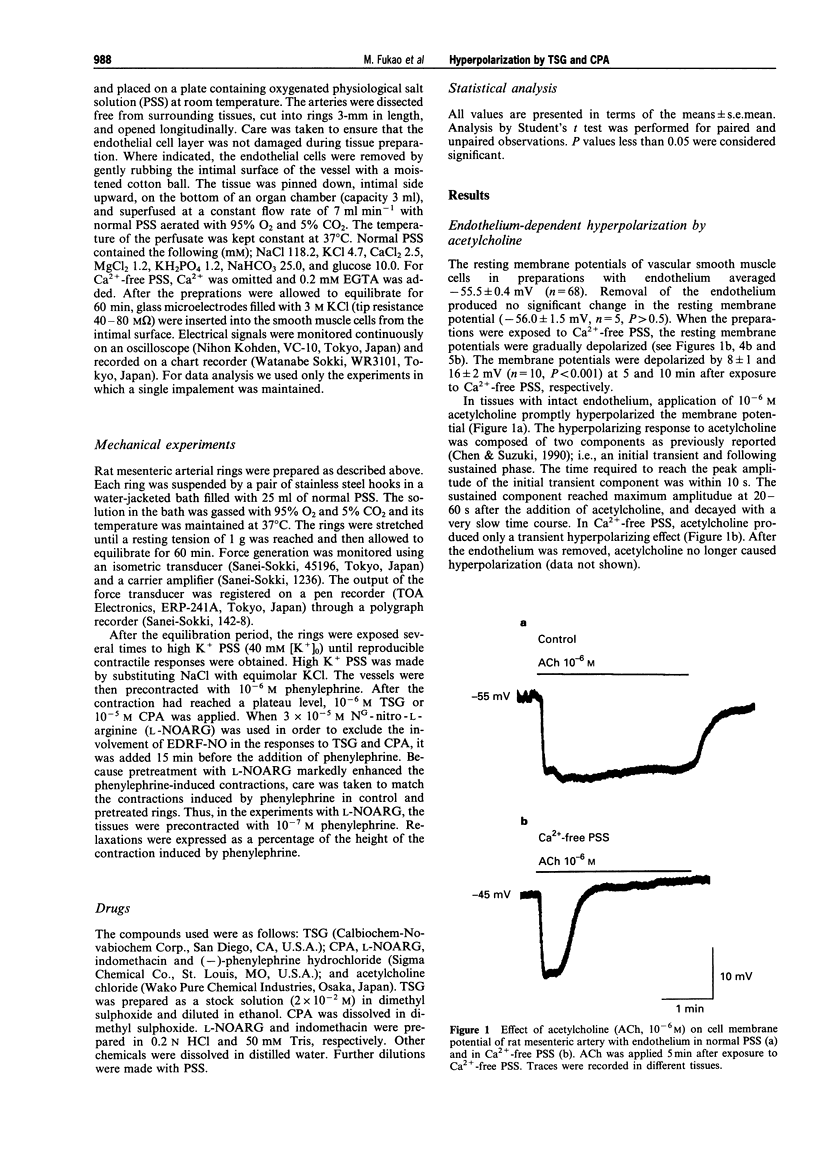
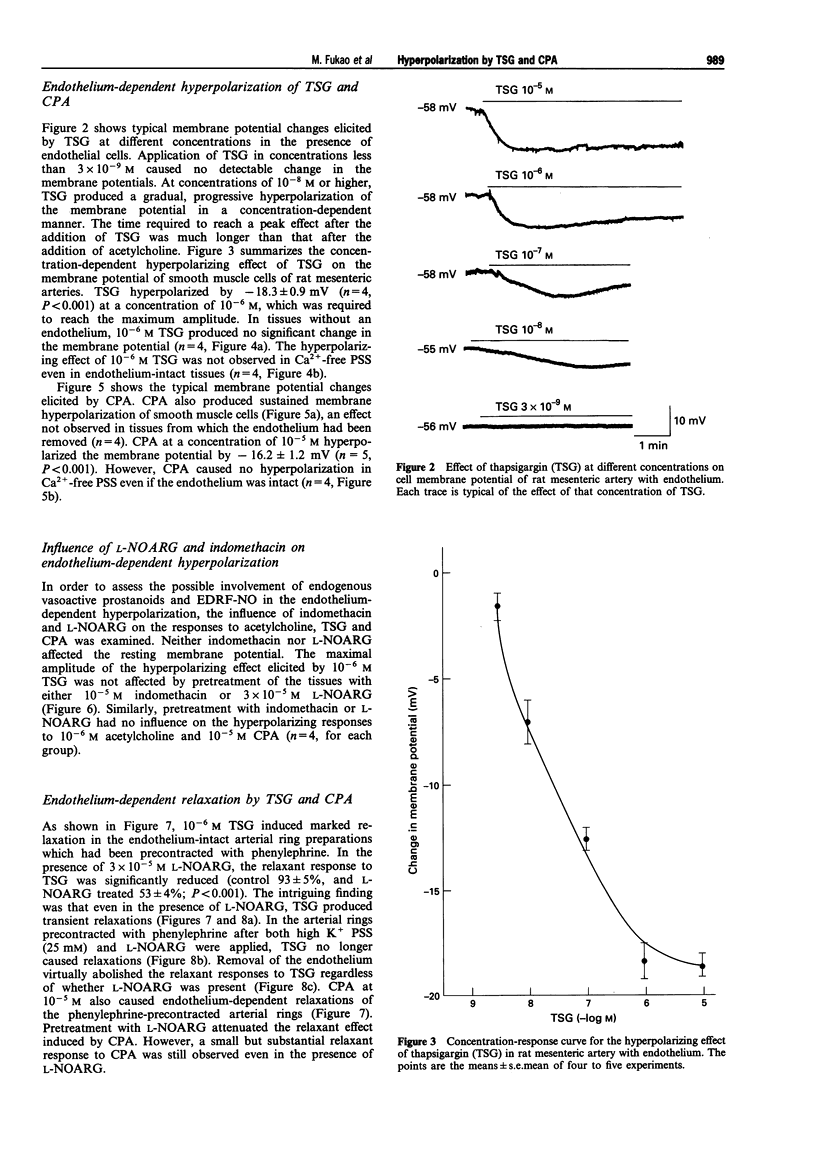
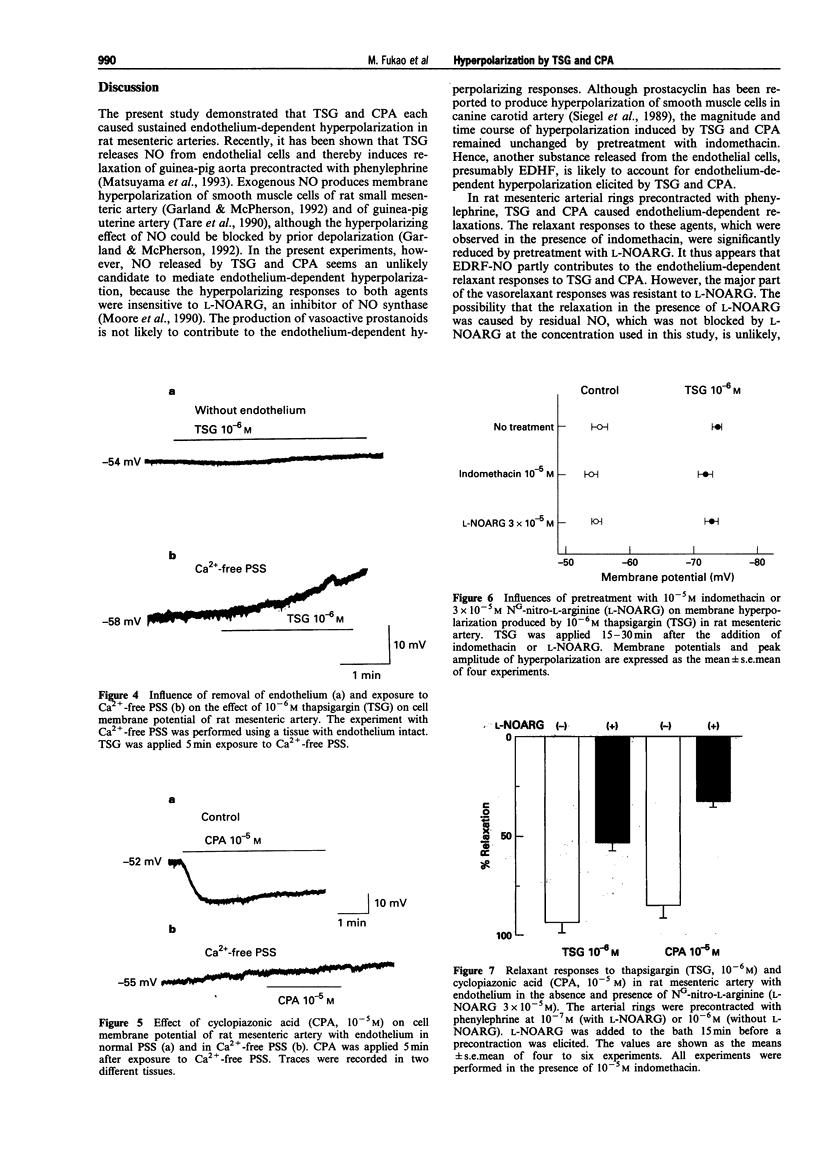
1. The present study was designed to determine whether putative, selective inhibitors of the Ca(2+)-pump ATPase of endoplasmic reticulum, thapsigargin (TSG) and cyclopiazonic acid (CPA), induce endothelium-dependent hyperpolarization in the rat isolated mesenteric artery. The membrane potentials of smooth muscle cells of main superior mesenteric arteries were measured by the microelectrode technique. 2. In tissues with endothelium, TSG (10(-8)-10(-5) M) caused sustained hyperpolarization in a concentration-dependent manner. In tissues without endothelium, TSG did not cause any change in membrane potential. CPA (10(-5) M) also hyperpolarized the smooth muscle membrane, an effect that was endothelium-dependent and long-lasting. 3. The hyperpolarizing responses to these agents were not affected by indomethacin or NG-nitro-L-arginine (L-NOARG). 4. In Ca(2+)-free medium, neither TSG nor CPA elicited hyperpolarization, in contrast to acetylcholine which generated a transient hyperpolarizing response. 5. In rings of mesenteric artery precontracted with phenylephrine, TSG and CPA produced endothelium-dependent relaxations. L-NOARG significantly inhibited the relaxations to these agents, but about 40-60% of the total relaxation was resistant to L-NOARG. The L-NOARG-resistant relaxations were abolished by potassium depolarization. 6. These results indicate that TSG and CPA can cause endothelium-dependent hyperpolarization in rat mesenteric artery possibly by releasing endothelium-derived hyperpolarizing factor and that membrane hyperpolarization can contribute to the endothelium-dependent relaxations to these agents. The mechanism of hyperpolarization may be related to increased Ca2+ influx into endothelial cells triggered by depletion of intracellular Ca2+ stores due to inhibition of endoplasmic reticulum Ca(2+)-pump ATPase activity.
Full text
PDF
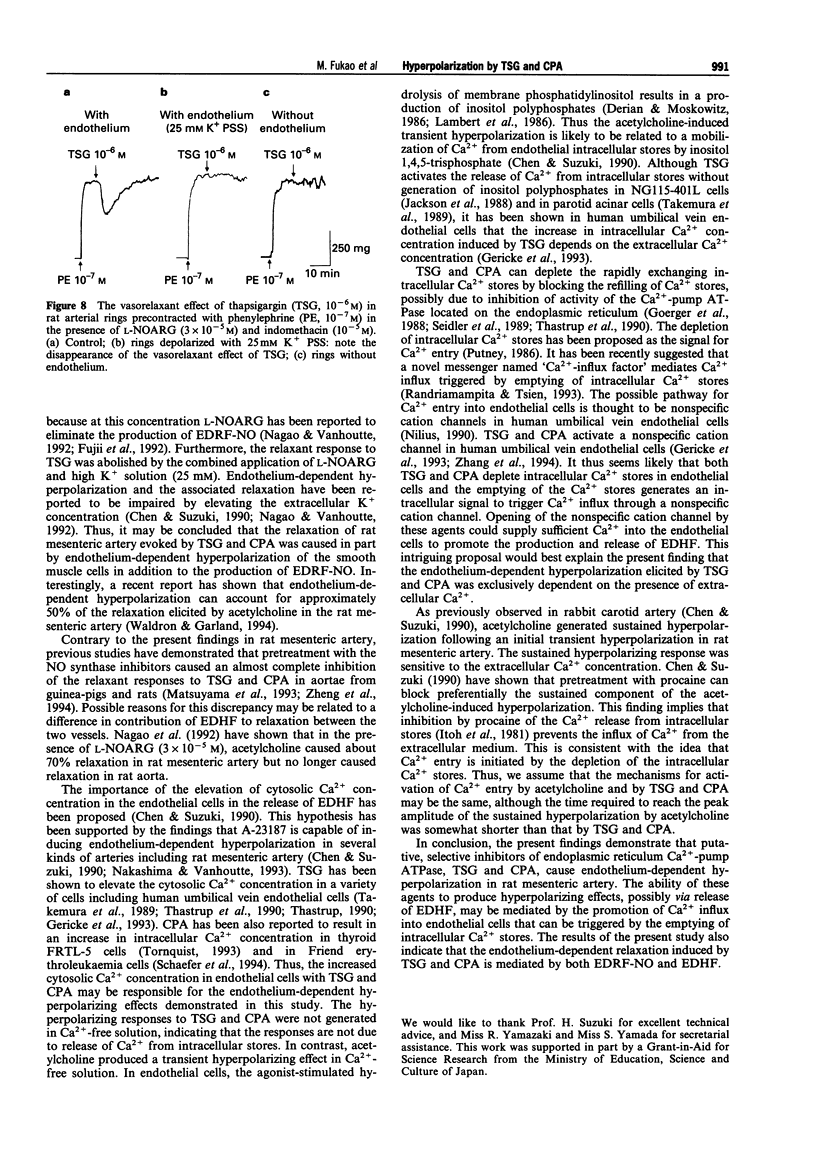

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bény J. L., Brunet P. C. Neither nitric oxide nor nitroglycerin accounts for all the characteristics of endothelially mediated vasodilatation of pig coronary arteries. Blood Vessels. 1988;25(6):308–311. [PubMed] [Google Scholar]
- Chen G. F., Suzuki H. Calcium dependency of the endothelium-dependent hyperpolarization in smooth muscle cells of the rabbit carotid artery. J Physiol. 1990 Feb;421:521–534. doi: 10.1113/jphysiol.1990.sp017959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G., Suzuki H., Weston A. H. Acetylcholine releases endothelium-derived hyperpolarizing factor and EDRF from rat blood vessels. Br J Pharmacol. 1988 Dec;95(4):1165–1174. doi: 10.1111/j.1476-5381.1988.tb11752.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derian C. K., Moskowitz M. A. Polyphosphoinositide hydrolysis in endothelial cells and carotid artery segments. Bradykinin-2 receptor stimulation is calcium-independent. J Biol Chem. 1986 Mar 15;261(8):3831–3837. [PubMed] [Google Scholar]
- Feletou M., Vanhoutte P. M. Endothelium-dependent hyperpolarization of canine coronary smooth muscle. Br J Pharmacol. 1988 Mar;93(3):515–524. doi: 10.1111/j.1476-5381.1988.tb10306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii K., Tominaga M., Ohmori S., Kobayashi K., Koga T., Takata Y., Fujishima M. Decreased endothelium-dependent hyperpolarization to acetylcholine in smooth muscle of the mesenteric artery of spontaneously hypertensive rats. Circ Res. 1992 Apr;70(4):660–669. doi: 10.1161/01.res.70.4.660. [DOI] [PubMed] [Google Scholar]
- Furchgott R. F., Vanhoutte P. M. Endothelium-derived relaxing and contracting factors. FASEB J. 1989 Jul;3(9):2007–2018. [PubMed] [Google Scholar]
- Furchgott R. F., Zawadzki J. V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980 Nov 27;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Garland C. J., McPherson G. A. Evidence that nitric oxide does not mediate the hyperpolarization and relaxation to acetylcholine in the rat small mesenteric artery. Br J Pharmacol. 1992 Feb;105(2):429–435. doi: 10.1111/j.1476-5381.1992.tb14270.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gericke M., Droogmans G., Nilius B. Thapsigargin discharges intracellular calcium stores and induces transmembrane currents in human endothelial cells. Pflugers Arch. 1993 Mar;422(6):552–557. doi: 10.1007/BF00374001. [DOI] [PubMed] [Google Scholar]
- Goeger D. E., Riley R. T., Dorner J. W., Cole R. J. Cyclopiazonic acid inhibition of the Ca2+-transport ATPase in rat skeletal muscle sarcoplasmic reticulum vesicles. Biochem Pharmacol. 1988 Mar 1;37(5):978–981. doi: 10.1016/0006-2952(88)90195-5. [DOI] [PubMed] [Google Scholar]
- Itoh T., Kuriyama H., Suzuki H. Excitation--contraction coupling in smooth muscle cells of the guinea-pig mesenteric artery. J Physiol. 1981 Dec;321:513–535. doi: 10.1113/jphysiol.1981.sp014000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson T. R., Patterson S. I., Thastrup O., Hanley M. R. A novel tumour promoter, thapsigargin, transiently increases cytoplasmic free Ca2+ without generation of inositol phosphates in NG115-401L neuronal cells. Biochem J. 1988 Jul 1;253(1):81–86. doi: 10.1042/bj2530081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert T. L., Kent R. S., Whorton A. R. Bradykinin stimulation of inositol polyphosphate production in porcine aortic endothelial cells. J Biol Chem. 1986 Nov 15;261(32):15288–15293. [PubMed] [Google Scholar]
- Lüscher T. F., Boulanger C. M., Dohi Y., Yang Z. H. Endothelium-derived contracting factors. Hypertension. 1992 Feb;19(2):117–130. doi: 10.1161/01.hyp.19.2.117. [DOI] [PubMed] [Google Scholar]
- Matsuyama S., Shuntoh H., Katayama S., Tanaka C. Thapsigargin induces an endothelium-dependent, intracellular calcium ion-dependent vasodilation in vitro. Life Sci. 1993;53(9):681–688. doi: 10.1016/0024-3205(93)90244-w. [DOI] [PubMed] [Google Scholar]
- Mikkelsen E. O., Thastrup O., Christensen S. B. Effects of thapsigargin in isolated rat thoracic aorta. Pharmacol Toxicol. 1988 Jan;62(1):7–11. doi: 10.1111/j.1600-0773.1988.tb01835.x. [DOI] [PubMed] [Google Scholar]
- Moncada S., Herman A. G., Higgs E. A., Vane J. R. Differential formation of prostacyclin (PGX or PGI2) by layers of the arterial wall. An explanation for the anti-thrombotic properties of vascular endothelium. Thromb Res. 1977 Sep;11(3):323–344. doi: 10.1016/0049-3848(77)90185-2. [DOI] [PubMed] [Google Scholar]
- Moore P. K., al-Swayeh O. A., Chong N. W., Evans R. A., Gibson A. L-NG-nitro arginine (L-NOARG), a novel, L-arginine-reversible inhibitor of endothelium-dependent vasodilatation in vitro. Br J Pharmacol. 1990 Feb;99(2):408–412. doi: 10.1111/j.1476-5381.1990.tb14717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers P. R., Minor R. L., Jr, Guerra R., Jr, Bates J. N., Harrison D. G. Vasorelaxant properties of the endothelium-derived relaxing factor more closely resemble S-nitrosocysteine than nitric oxide. Nature. 1990 May 10;345(6271):161–163. doi: 10.1038/345161a0. [DOI] [PubMed] [Google Scholar]
- Nagao T., Illiano S., Vanhoutte P. M. Heterogeneous distribution of endothelium-dependent relaxations resistant to NG-nitro-L-arginine in rats. Am J Physiol. 1992 Oct;263(4 Pt 2):H1090–H1094. doi: 10.1152/ajpheart.1992.263.4.H1090. [DOI] [PubMed] [Google Scholar]
- Nagao T., Vanhoutte P. M. Hyperpolarization as a mechanism for endothelium-dependent relaxations in the porcine coronary artery. J Physiol. 1992 Jan;445:355–367. doi: 10.1113/jphysiol.1992.sp018928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima M., Vanhoutte P. M. Endothelin-1 and -3 cause endothelium-dependent hyperpolarization in the rat mesenteric artery. Am J Physiol. 1993 Dec;265(6 Pt 2):H2137–H2141. doi: 10.1152/ajpheart.1993.265.6.H2137. [DOI] [PubMed] [Google Scholar]
- Nilius B. Permeation properties of a non-selective cation channel in human vascular endothelial cells. Pflugers Arch. 1990 Jul;416(5):609–611. doi: 10.1007/BF00382697. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Ferrige A. G., Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987 Jun 11;327(6122):524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Putney J. W., Jr A model for receptor-regulated calcium entry. Cell Calcium. 1986 Feb;7(1):1–12. doi: 10.1016/0143-4160(86)90026-6. [DOI] [PubMed] [Google Scholar]
- Randriamampita C., Tsien R. Y. Emptying of intracellular Ca2+ stores releases a novel small messenger that stimulates Ca2+ influx. Nature. 1993 Aug 26;364(6440):809–814. doi: 10.1038/364809a0. [DOI] [PubMed] [Google Scholar]
- Schaefer A., Magócsi M., Stöcker U., Kósa F., Marquardt H. Early transient suppression of c-myb mRNA levels and induction of differentiation in Friend erythroleukemia cells by the [Ca2+]i-increasing agents cyclopiazonic acid and thapsigargin. J Biol Chem. 1994 Mar 25;269(12):8786–8791. [PubMed] [Google Scholar]
- Seidler N. W., Jona I., Vegh M., Martonosi A. Cyclopiazonic acid is a specific inhibitor of the Ca2+-ATPase of sarcoplasmic reticulum. J Biol Chem. 1989 Oct 25;264(30):17816–17823. [PubMed] [Google Scholar]
- Siegel G., Carl A., Adler A., Stock G. Effect of the prostacyclin analogue iloprost on K+ permeability in the smooth muscle cells of the canine carotid artery. Eicosanoids. 1989;2(4):213–222. [PubMed] [Google Scholar]
- Suzuki H., Chen G., Yamamoto Y. Endothelium-derived hyperpolarizing factor (EDHF). Jpn Circ J. 1992 Feb;56(2):170–174. doi: 10.1253/jcj.56.170. [DOI] [PubMed] [Google Scholar]
- Takemura H., Hughes A. R., Thastrup O., Putney J. W., Jr Activation of calcium entry by the tumor promoter thapsigargin in parotid acinar cells. Evidence that an intracellular calcium pool and not an inositol phosphate regulates calcium fluxes at the plasma membrane. J Biol Chem. 1989 Jul 25;264(21):12266–12271. [PubMed] [Google Scholar]
- Tare M., Parkington H. C., Coleman H. A., Neild T. O., Dusting G. J. Hyperpolarization and relaxation of arterial smooth muscle caused by nitric oxide derived from the endothelium. Nature. 1990 Jul 5;346(6279):69–71. doi: 10.1038/346069a0. [DOI] [PubMed] [Google Scholar]
- Taylor S. G., Weston A. H. Endothelium-derived hyperpolarizing factor: a new endogenous inhibitor from the vascular endothelium. Trends Pharmacol Sci. 1988 Aug;9(8):272–274. doi: 10.1016/0165-6147(88)90003-x. [DOI] [PubMed] [Google Scholar]
- Thastrup O., Cullen P. J., Drøbak B. K., Hanley M. R., Dawson A. P. Thapsigargin, a tumor promoter, discharges intracellular Ca2+ stores by specific inhibition of the endoplasmic reticulum Ca2(+)-ATPase. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2466–2470. doi: 10.1073/pnas.87.7.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thastrup O. Role of Ca2(+)-ATPases in regulation of cellular Ca2+ signalling, as studied with the selective microsomal Ca2(+)-ATPase inhibitor, thapsigargin. Agents Actions. 1990 Jan;29(1-2):8–15. doi: 10.1007/BF01964706. [DOI] [PubMed] [Google Scholar]
- Törnquist K. Activation of calcium entry by cyclopiazonic acid in thyroid FRTL-5 cells. Cell Calcium. 1993 May;14(5):411–417. doi: 10.1016/0143-4160(93)90045-8. [DOI] [PubMed] [Google Scholar]
- Waldron G. J., Garland C. J. Contribution of both nitric oxide and a change in membrane potential to acetylcholine-induced relaxation in the rat small mesenteric artery. Br J Pharmacol. 1994 Jul;112(3):831–836. doi: 10.1111/j.1476-5381.1994.tb13154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Inazu M., Weir B., Buchanan M., Daniel E. Cyclopiazonic acid stimulates Ca2+ influx through non-specific cation channels in endothelial cells. Eur J Pharmacol. 1994 Jan 14;251(2-3):119–125. doi: 10.1016/0014-2999(94)90391-3. [DOI] [PubMed] [Google Scholar]
- Zheng X. F., Kwan C. Y., Daniel E. E. Role of intracellular Ca2+ in EDRF release in rat aorta. J Vasc Res. 1994 Jan-Feb;31(1):18–24. doi: 10.1159/000159027. [DOI] [PubMed] [Google Scholar]