Abstract
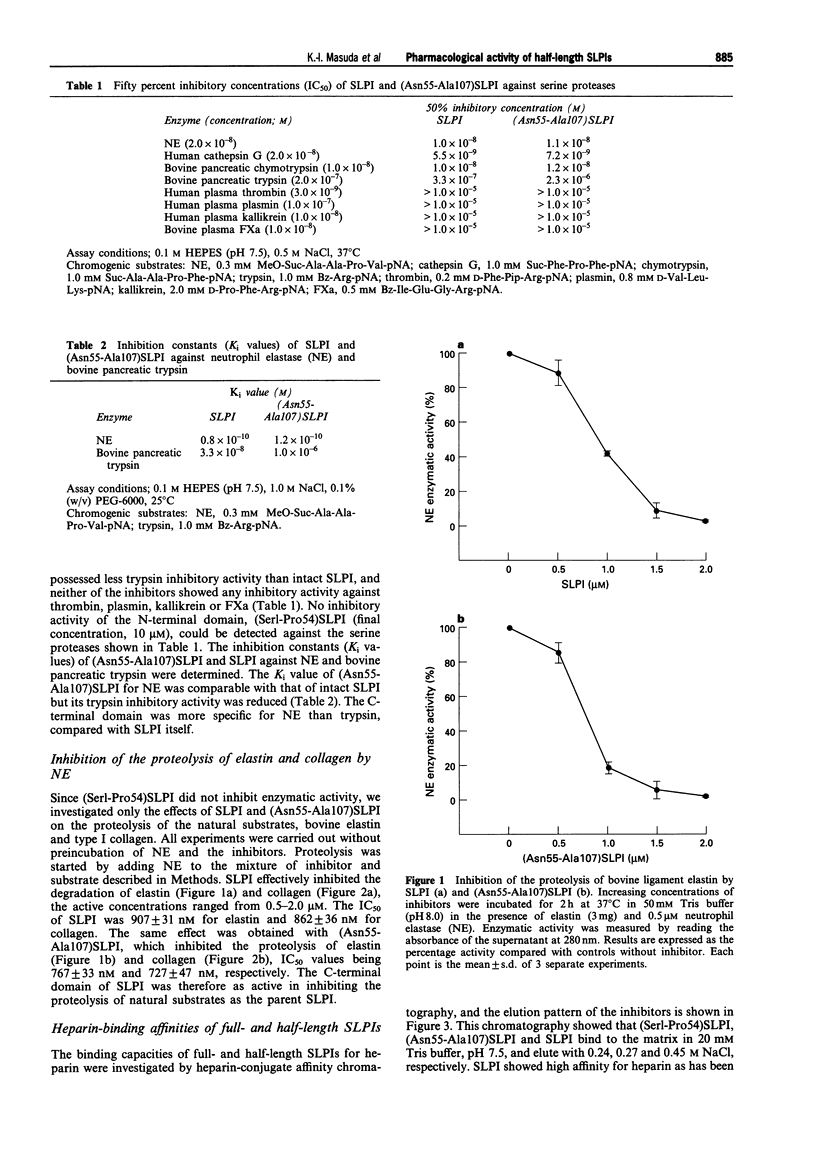
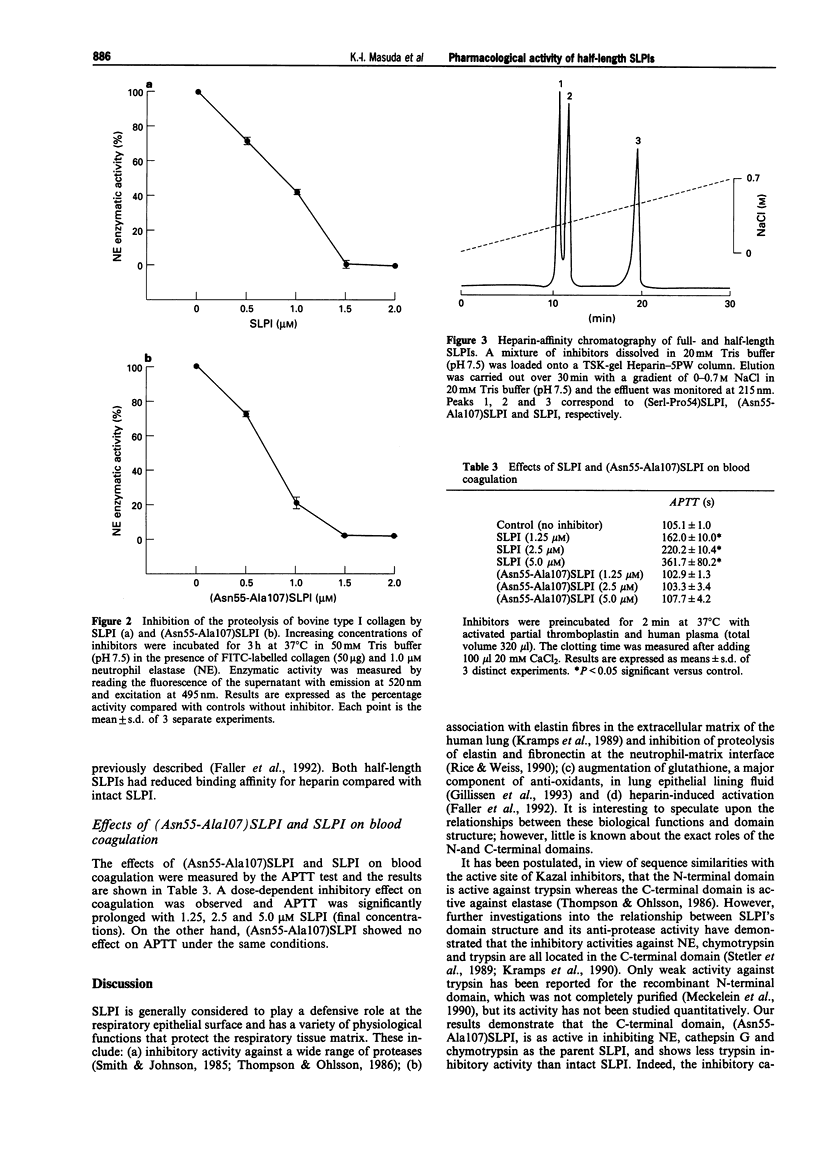
1. In order to characterize the physiological functions of the domain structure of secretory leukoprotease inhibitor (SLPI), the biological capacities of half-length SLPIs, (Ser1-Pro54)SLPI and (Asn55-Ala107)SLPI, were investigated and compared with those of full-length SLPI. 2. The activities of these inhibitors against several serine proteases were determined using synthetic chromogenic substrates. The inhibitory capacity of the C-terminal domain, (Asn55-Ala107)SLPI, was as strong as that of full-length SLPI against human neutrophil elastase (NE), cathepsin G and chymotrypsin. It possessed less trypsin inhibitory activity than intact SLPI. For the N-terminal domain of SLPI, (Ser1-Pro54)SLPI, no inhibitory activity could be detected against the serine proteases tested in this study. 3. The inhibitory activity of (Asn55-Ala107)SLPI against the proteolysis of the natural substrates elastin and collagen by NE was comparable with that of full-SLPI (elastin, IC50 = 907 +/- 31 nM for SLPI, 767 +/- 33 nM for (Asn55-Ala107)SLPI; collagen, IC50 = 862 +/- 36 nM for SLPI, 727 +/- 47 nM for (Asn55-Ala107)SLPI). 4. The binding affinities of full- and half-length SLPIs for heparin were measured by affinity column chromatography. Full-length SLPI showed high affinity for heparin while the binding capacities of both half-length SLPIs were lower. (Concentration of NaCl for elution, 0.45 M for SLPI, 0.24 M for (Ser1-Pro54)SLPI, 0.27 M for (Asn55-Ala107)SLPI). 5. The effects of full-SLPI and (Asn55-Ala107)SLPI on blood coagulation were measured using the activated partial thromboplastin time (APTT).(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beatty K., Bieth J., Travis J. Kinetics of association of serine proteinases with native and oxidized alpha-1-proteinase inhibitor and alpha-1-antichymotrypsin. J Biol Chem. 1980 May 10;255(9):3931–3934. [PubMed] [Google Scholar]
- Boudier C., Holle C., Bieth J. G. Stimulation of the elastolytic activity of leukocyte elastase by leukocyte cathepsin G. J Biol Chem. 1981 Oct 25;256(20):10256–10258. [PubMed] [Google Scholar]
- Campbell E. J., Senior R. M., Welgus H. G. Extracellular matrix injury during lung inflammation. Chest. 1987 Jul;92(1):161–167. doi: 10.1378/chest.92.1.161. [DOI] [PubMed] [Google Scholar]
- Crystal R. G., Brantly M. L., Hubbard R. C., Curiel D. T., States D. J., Holmes M. D. The alpha 1-antitrypsin gene and its mutations. Clinical consequences and strategies for therapy. Chest. 1989 Jan;95(1):196–208. doi: 10.1378/chest.95.1.196. [DOI] [PubMed] [Google Scholar]
- De Water R., Willems L. N., Van Muijen G. N., Franken C., Fransen J. A., Dijkman J. H., Kramps J. A. Ultrastructural localization of bronchial antileukoprotease in central and peripheral human airways by a gold-labeling technique using monoclonal antibodies. Am Rev Respir Dis. 1986 May;133(5):882–890. [PubMed] [Google Scholar]
- Faller B., Mely Y., Gerard D., Bieth J. G. Heparin-induced conformational change and activation of mucus proteinase inhibitor. Biochemistry. 1992 Sep 8;31(35):8285–8290. doi: 10.1021/bi00150a023. [DOI] [PubMed] [Google Scholar]
- Franken C., Meijer C. J., Dijkman J. H. Tissue distribution of antileukoprotease and lysozyme in humans. J Histochem Cytochem. 1989 Apr;37(4):493–498. doi: 10.1177/37.4.2926127. [DOI] [PubMed] [Google Scholar]
- Fulcher C. A., Zimmerman T. S. Characterization of the human factor VIII procoagulant protein with a heterologous precipitating antibody. Proc Natl Acad Sci U S A. 1982 Mar;79(5):1648–1652. doi: 10.1073/pnas.79.5.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillissen A., Birrer P., McElvaney N. G., Buhl R., Vogelmeier C., Hoyt R. F., Jr, Hubbard R. C., Crystal R. G. Recombinant secretory leukoprotease inhibitor augments glutathione levels in lung epithelial lining fluid. J Appl Physiol (1985) 1993 Aug;75(2):825–832. doi: 10.1152/jappl.1993.75.2.825. [DOI] [PubMed] [Google Scholar]
- Grütter M. G., Fendrich G., Huber R., Bode W. The 2.5 A X-ray crystal structure of the acid-stable proteinase inhibitor from human mucous secretions analysed in its complex with bovine alpha-chymotrypsin. EMBO J. 1988 Feb;7(2):345–351. doi: 10.1002/j.1460-2075.1988.tb02819.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson P. J. A linear equation that describes the steady-state kinetics of enzymes and subcellular particles interacting with tightly bound inhibitors. Biochem J. 1972 Apr;127(2):321–333. doi: 10.1042/bj1270321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikehara M., Ohtsuka E., Tokunaga T., Taniyama Y., Iwai S., Kitano K., Miyamoto S., Ohgi T., Sakuragawa Y., Fujiyama K. Synthesis of a gene for human growth hormone and its expression in Escherichia coli. Proc Natl Acad Sci U S A. 1984 Oct;81(19):5956–5960. doi: 10.1073/pnas.81.19.5956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janoff A. Elastases and emphysema. Current assessment of the protease-antiprotease hypothesis. Am Rev Respir Dis. 1985 Aug;132(2):417–433. doi: 10.1164/arrd.1985.132.2.417. [DOI] [PubMed] [Google Scholar]
- Janusz M. J., Doherty N. S. Degradation of cartilage matrix proteoglycan by human neutrophils involves both elastase and cathepsin G. J Immunol. 1991 Jun 1;146(11):3922–3928. [PubMed] [Google Scholar]
- Keiser H., Greenwald R. A., Feinstein G., Janoff A. Degradation of cartilage proteoglycan by human leukocyte granule neutral proteases--a model of joint injury. II. Degradation of isolated bovine nasal cartilage proteoglycan. J Clin Invest. 1976 Mar;57(3):625–632. doi: 10.1172/JCI108318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramps J. A., Franken C., Meijer C. J., Dijkman J. H. Localization of low molecular weight protease inhibitor in serous secretory cells of the respiratory tract. J Histochem Cytochem. 1981 Jun;29(6):712–719. doi: 10.1177/29.6.6788837. [DOI] [PubMed] [Google Scholar]
- Kramps J. A., Te Boekhorst A. H., Fransen J. A., Ginsel L. A., Dijkman J. H. Antileukoprotease is associated with elastin fibers in the extracellular matrix of the human lung. An immunoelectron microscopic study. Am Rev Respir Dis. 1989 Aug;140(2):471–476. doi: 10.1164/ajrccm/140.2.471. [DOI] [PubMed] [Google Scholar]
- Kramps J. A., van Twisk C., Appelhans H., Meckelein B., Nikiforov T., Dijkman J. H. Proteinase inhibitory activities of antileukoprotease are represented by its second COOH-terminal domain. Biochim Biophys Acta. 1990 Apr 19;1038(2):178–185. doi: 10.1016/0167-4838(90)90202-q. [DOI] [PubMed] [Google Scholar]
- Lucey E. C., Stone P. J., Ciccolella D. E., Breuer R., Christensen T. G., Thompson R. C., Snider G. L. Recombinant human secretory leukocyte-protease inhibitor: in vitro properties, and amelioration of human neutrophil elastase-induced emphysema and secretory cell metaplasia in the hamster. J Lab Clin Med. 1990 Feb;115(2):224–232. [PubMed] [Google Scholar]
- Mainardi C. L., Hasty D. L., Seyer J. M., Kang A. H. Specific cleavage of human type III collagen by human polymorphonuclear leukocyte elastase. J Biol Chem. 1980 Dec 25;255(24):12006–12010. [PubMed] [Google Scholar]
- McDonald J. A., Kelley D. G. Degradation of fibronectin by human leukocyte elastase. Release of biologically active fragments. J Biol Chem. 1980 Sep 25;255(18):8848–8858. [PubMed] [Google Scholar]
- Meckelein B., Nikiforov T., Clemen A., Appelhans H. The location of inhibitory specificities in human mucus proteinase inhibitor (MPI): separate expression of the COOH-terminal domain yields an active inhibitor of three different proteinases. Protein Eng. 1990 Jan;3(3):215–220. doi: 10.1093/protein/3.3.215. [DOI] [PubMed] [Google Scholar]
- Meyer K. C., Lewandoski J. R., Zimmerman J. J., Nunley D., Calhoun W. J., Dopico G. A. Human neutrophil elastase and elastase/alpha 1-antiprotease complex in cystic fibrosis. Comparison with interstitial lung disease and evaluation of the effect of intravenously administered antibiotic therapy. Am Rev Respir Dis. 1991 Sep;144(3 Pt 1):580–585. doi: 10.1164/ajrccm/144.3_Pt_1.580. [DOI] [PubMed] [Google Scholar]
- Piccioni P. D., Kramps J. A., Rudolphus A., Bulgheroni A., Luisetti M. Proteinase/proteinase inhibitor imbalance in sputum sol phases from patients with chronic obstructive pulmonary disease. Suggestions for a key role played by antileukoprotease. Chest. 1992 Nov;102(5):1470–1476. doi: 10.1378/chest.102.5.1470. [DOI] [PubMed] [Google Scholar]
- Renesto P., Balloy V., Kamimura T., Masuda K., Imaizumi A., Chignard M. Inhibition by recombinant SLPI and half-SLPI (Asn55-Ala107) of elastase and cathepsin G activities: consequence for neutrophil-platelet cooperation. Br J Pharmacol. 1993 Apr;108(4):1100–1106. doi: 10.1111/j.1476-5381.1993.tb13511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice W. G., Weiss S. J. Regulation of proteolysis at the neutrophil-substrate interface by secretory leukoprotease inhibitor. Science. 1990 Jul 13;249(4965):178–181. doi: 10.1126/science.2371565. [DOI] [PubMed] [Google Scholar]
- Rudolphus A., Kramps J. A., Dijkman J. H. Effect of human antileucoprotease on experimental emphysema. Eur Respir J. 1991 Jan;4(1):31–39. [PubMed] [Google Scholar]
- Sallenave J. M., Silva A., Marsden M. E., Ryle A. P. Secretion of mucus proteinase inhibitor and elafin by Clara cell and type II pneumocyte cell lines. Am J Respir Cell Mol Biol. 1993 Feb;8(2):126–133. doi: 10.1165/ajrcmb/8.2.126. [DOI] [PubMed] [Google Scholar]
- Smith C. E., Johnson D. A. Human bronchial leucocyte proteinase inhibitor. Rapid isolation and kinetic analysis with human leucocyte proteinases. Biochem J. 1985 Jan 15;225(2):463–472. doi: 10.1042/bj2250463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snider G. L., Lucey E. C., Christensen T. G., Stone P. J., Calore J. D., Catanese A., Franzblau C. Emphysema and bronchial secretory cell metaplasia induced in hamsters by human neutrophil products. Am Rev Respir Dis. 1984 Jan;129(1):155–160. doi: 10.1164/arrd.1984.129.1.155. [DOI] [PubMed] [Google Scholar]
- Thompson R. C., Ohlsson K. Isolation, properties, and complete amino acid sequence of human secretory leukocyte protease inhibitor, a potent inhibitor of leukocyte elastase. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6692–6696. doi: 10.1073/pnas.83.18.6692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis J., Fritz H. Potential problems in designing elastase inhibitors for therapy. Am Rev Respir Dis. 1991 Jun;143(6):1412–1415. doi: 10.1164/ajrccm/143.6.1412. [DOI] [PubMed] [Google Scholar]
- Vogelmeier C., Buhl R., Hoyt R. F., Wilson E., Fells G. A., Hubbard R. C., Schnebli H. P., Thompson R. C., Crystal R. G. Aerosolization of recombinant SLPI to augment antineutrophil elastase protection of pulmonary epithelium. J Appl Physiol (1985) 1990 Nov;69(5):1843–1848. doi: 10.1152/jappl.1990.69.5.1843. [DOI] [PubMed] [Google Scholar]
- Vogelmeier C., Hubbard R. C., Fells G. A., Schnebli H. P., Thompson R. C., Fritz H., Crystal R. G. Anti-neutrophil elastase defense of the normal human respiratory epithelial surface provided by the secretory leukoprotease inhibitor. J Clin Invest. 1991 Feb;87(2):482–488. doi: 10.1172/JCI115021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wewers M. D., Casolaro M. A., Sellers S. E., Swayze S. C., McPhaul K. M., Wittes J. T., Crystal R. G. Replacement therapy for alpha 1-antitrypsin deficiency associated with emphysema. N Engl J Med. 1987 Apr 23;316(17):1055–1062. doi: 10.1056/NEJM198704233161704. [DOI] [PubMed] [Google Scholar]


