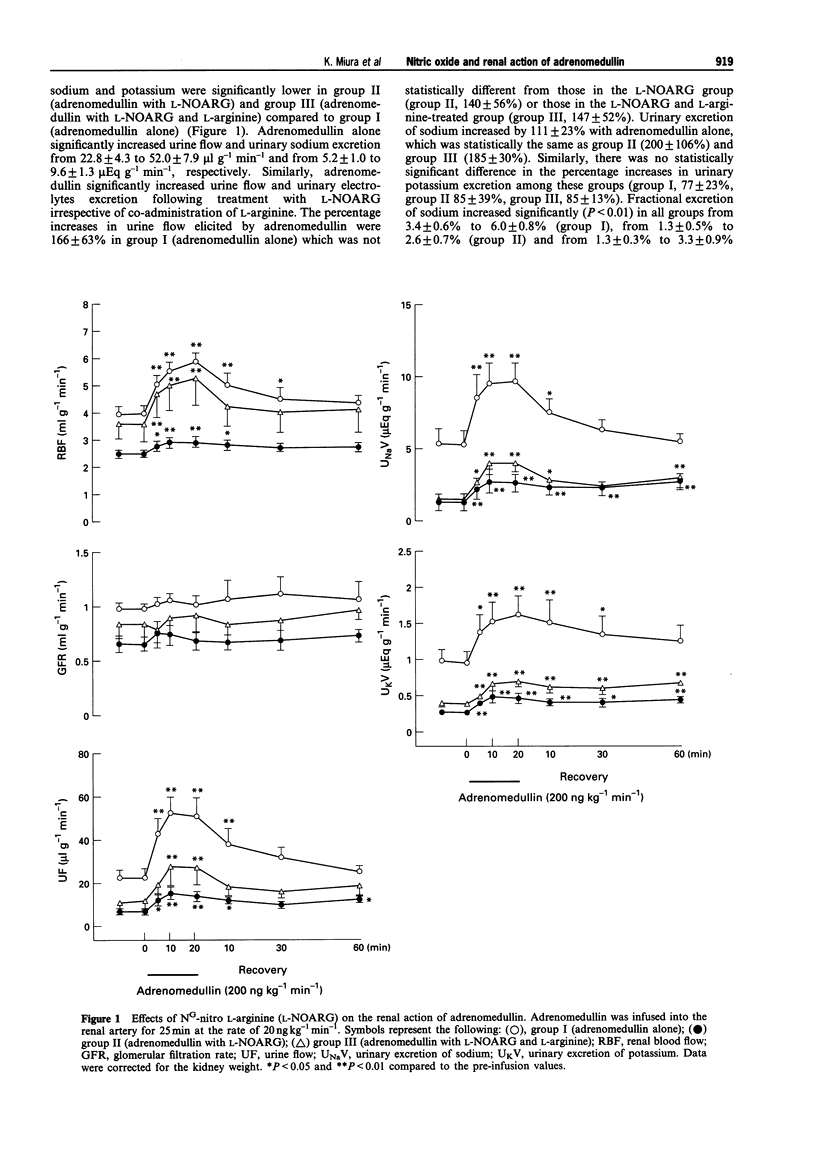
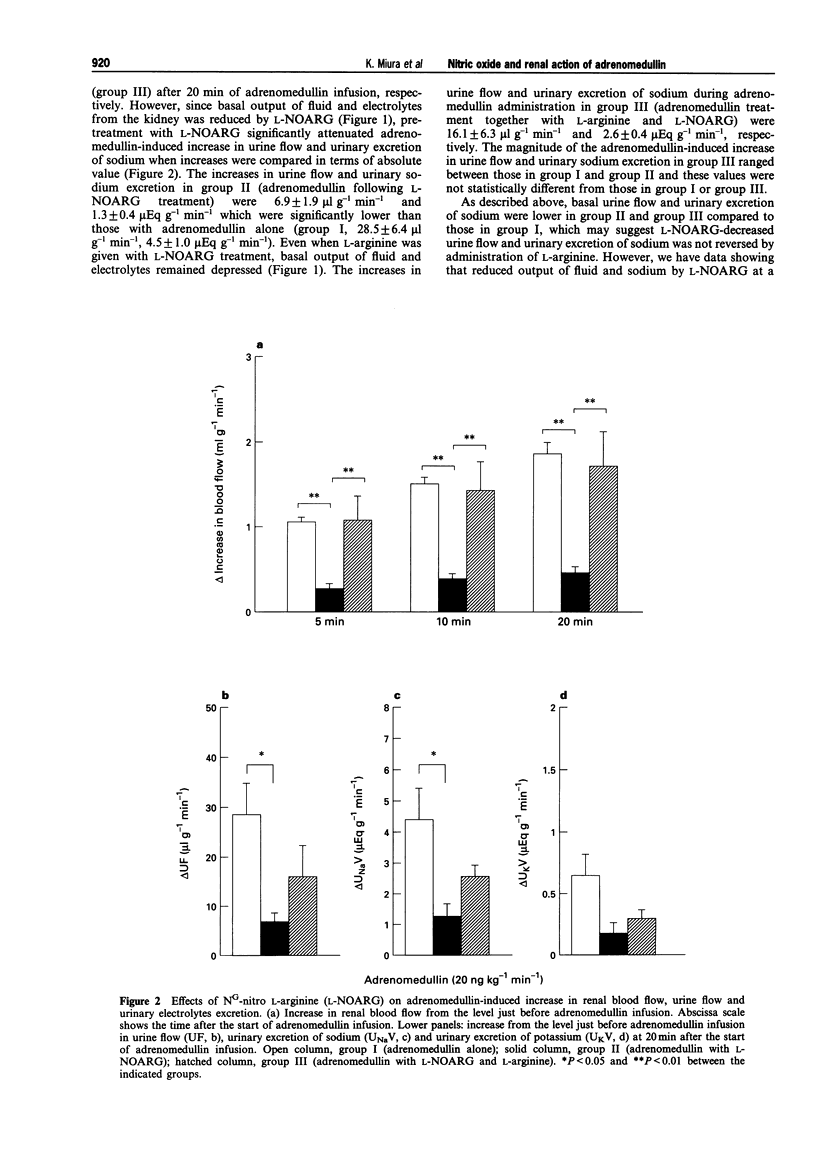
Abstract
1. The present study was conducted in order to elucidate the in vivo contribution of nitric oxide (NO) and the glibenclamide-sensitive potassium channel in the renal action of adrenomedullin in anaesthetized dogs. 2. Intrarenal arterial infusion of adrenomedullin (20 ng kg-1 min-1) elicited a pronounced increase in renal blood flow with no changes in systemic blood pressure. The renal vasodilator action of adrenomedullin was markedly attenuated by pretreatment with NG-nitro L-arginine (L-NOARG), but this was reversed by continuous infusion of L-arginine. 3. Pretreatment with glibenclamide almost completely blocked the renal vasodilatation induced by lemakalim, but had no effect on the renal vasodilator and diuretic action of adrenomedullin. 4. Intrarenal arterial infusion of adrenomedullin induced diuresis and natriuresis. Diuretic and natriuretic action of adrenomedullin was also attenuated by L-NOARG. L-Arginine partly reversed the effect of L-NOARG and adrenomedullin-induced diuresis and natriuresis. 5. These data indicate that the in vivo renal vasodilator action of adrenomedullin is mediated by the release of NO. The glibenclamide-sensitive potassium channel is not involved in the renal action of adrenomedullin, at least, not in anaesthetized dogs. Since the inhibition of L-NOARG of adrenomedullin-induced diuresis occurred concomitantly with the attenuation of the renal vasodilator action of adrenomedullin, direct involvement of NO in adrenomedullin-induced diuresis remains to be established.
Full text
PDF

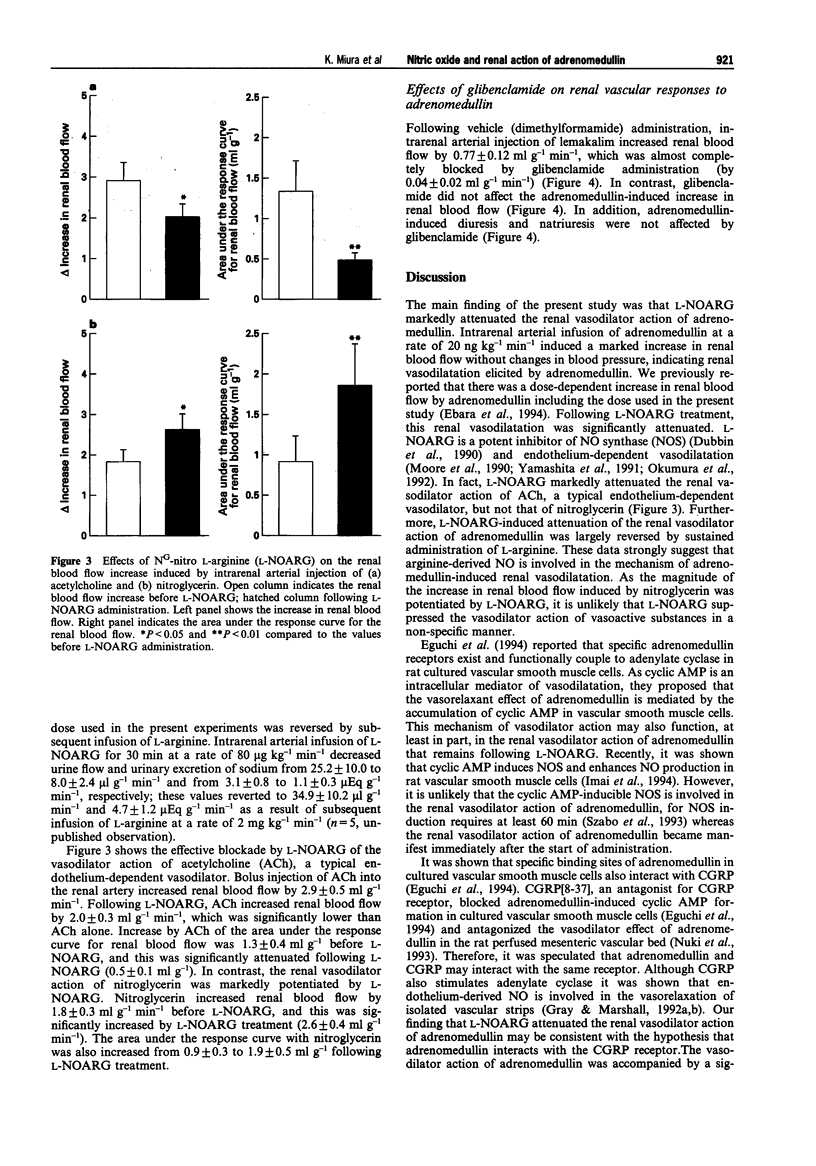
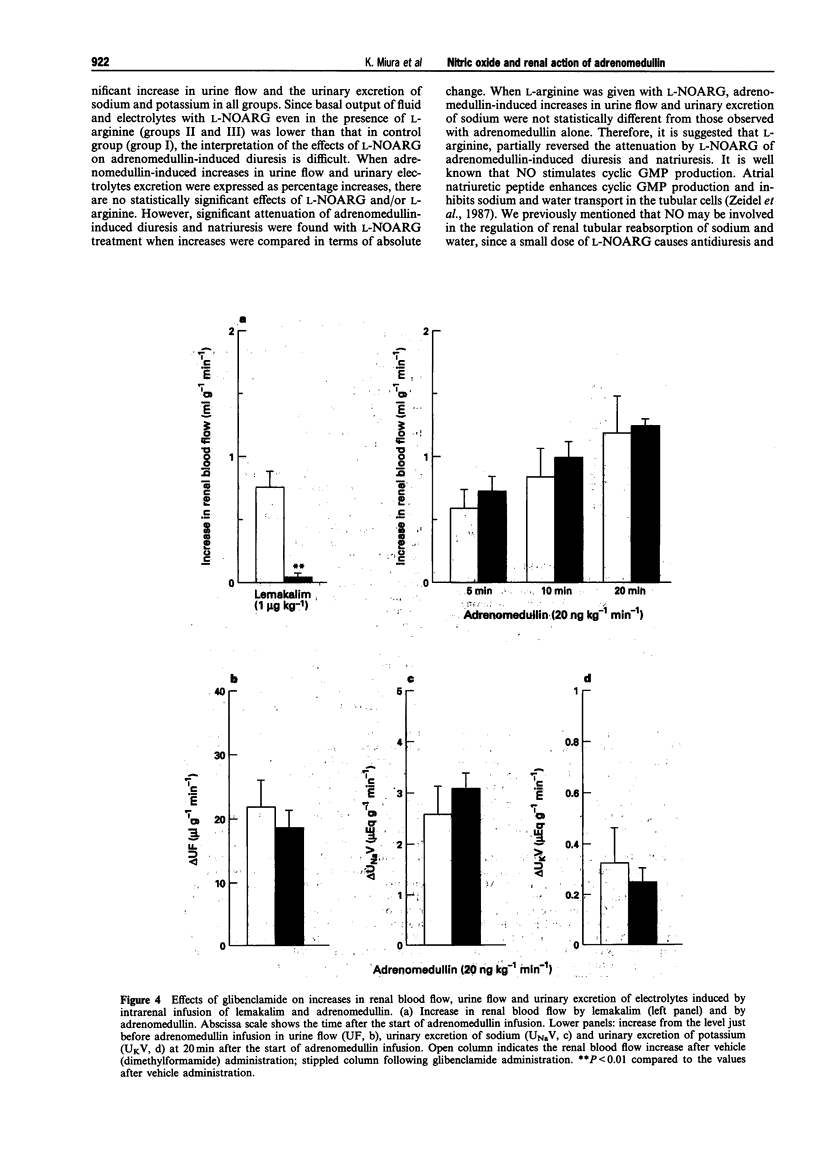


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baylis C., Deen W. M., Myers B. D., Brenner B. M. Effects of some vasodilator drugs on transcapillary fluid exchange in renal cortex. Am J Physiol. 1976 Apr;230(4):1148–1158. doi: 10.1152/ajplegacy.1976.230.4.1148. [DOI] [PubMed] [Google Scholar]
- Brain S. D., Williams T. J., Tippins J. R., Morris H. R., MacIntyre I. Calcitonin gene-related peptide is a potent vasodilator. Nature. 1985 Jan 3;313(5997):54–56. doi: 10.1038/313054a0. [DOI] [PubMed] [Google Scholar]
- Dubbin P. N., Zambetis M., Dusting G. J. Inhibition of endothelial nitric oxide biosynthesis by N-nitro-L-arginine. Clin Exp Pharmacol Physiol. 1990 Apr;17(4):281–286. doi: 10.1111/j.1440-1681.1990.tb01321.x. [DOI] [PubMed] [Google Scholar]
- Ebara T., Miura K., Okumura M., Matsuura T., Kim S., Yukimura T., Iwao H. Effect of adrenomedullin on renal hemodynamics and functions in dogs. Eur J Pharmacol. 1994 Sep 22;263(1-2):69–73. doi: 10.1016/0014-2999(94)90524-x. [DOI] [PubMed] [Google Scholar]
- Eguchi S., Hirata Y., Kano H., Sato K., Watanabe Y., Watanabe T. X., Nakajima K., Sakakibara S., Marumo F. Specific receptors for adrenomedullin in cultured rat vascular smooth muscle cells. FEBS Lett. 1994 Mar 7;340(3):226–230. doi: 10.1016/0014-5793(94)80143-6. [DOI] [PubMed] [Google Scholar]
- Gray D. W., Marshall I. Human alpha-calcitonin gene-related peptide stimulates adenylate cyclase and guanylate cyclase and relaxes rat thoracic aorta by releasing nitric oxide. Br J Pharmacol. 1992 Nov;107(3):691–696. doi: 10.1111/j.1476-5381.1992.tb14508.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray D. W., Marshall I. Nitric oxide synthesis inhibitors attenuate calcitonin gene-related peptide endothelium-dependent vasorelaxation in rat aorta. Eur J Pharmacol. 1992 Feb 25;212(1):37–42. doi: 10.1016/0014-2999(92)90069-g. [DOI] [PubMed] [Google Scholar]
- Imai T., Hirata Y., Kanno K., Marumo F. Induction of nitric oxide synthase by cyclic AMP in rat vascular smooth muscle cells. J Clin Invest. 1994 Feb;93(2):543–549. doi: 10.1172/JCI117005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizaka Y., Ishizaka Y., Tanaka M., Kitamura K., Kangawa K., Minamino N., Matsuo H., Eto T. Adrenomedullin stimulates cyclic AMP formation in rat vascular smooth muscle cells. Biochem Biophys Res Commun. 1994 Apr 15;200(1):642–646. doi: 10.1006/bbrc.1994.1496. [DOI] [PubMed] [Google Scholar]
- Kitamura K., Kangawa K., Kawamoto M., Ichiki Y., Nakamura S., Matsuo H., Eto T. Adrenomedullin: a novel hypotensive peptide isolated from human pheochromocytoma. Biochem Biophys Res Commun. 1993 Apr 30;192(2):553–560. doi: 10.1006/bbrc.1993.1451. [DOI] [PubMed] [Google Scholar]
- Kitamura K., Sakata J., Kangawa K., Kojima M., Matsuo H., Eto T. Cloning and characterization of cDNA encoding a precursor for human adrenomedullin. Biochem Biophys Res Commun. 1993 Jul 30;194(2):720–725. doi: 10.1006/bbrc.1993.1881. [DOI] [PubMed] [Google Scholar]
- Kubota M., Moseley J. M., Butera L., Dusting G. J., MacDonald P. S., Martin T. J. Calcitonin gene-related peptide stimulates cyclic AMP formation in rat aortic smooth muscle cells. Biochem Biophys Res Commun. 1985 Oct 15;132(1):88–94. doi: 10.1016/0006-291x(85)90992-1. [DOI] [PubMed] [Google Scholar]
- Majid D. S., Williams A., Navar L. G. Inhibition of nitric oxide synthesis attenuates pressure-induced natriuretic responses in anesthetized dogs. Am J Physiol. 1993 Jan;264(1 Pt 2):F79–F87. doi: 10.1152/ajprenal.1993.264.1.F79. [DOI] [PubMed] [Google Scholar]
- Moore P. K., al-Swayeh O. A., Chong N. W., Evans R. A., Gibson A. L-NG-nitro arginine (L-NOARG), a novel, L-arginine-reversible inhibitor of endothelium-dependent vasodilatation in vitro. Br J Pharmacol. 1990 Feb;99(2):408–412. doi: 10.1111/j.1476-5381.1990.tb14717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson M. T., Huang Y., Brayden J. E., Hescheler J., Standen N. B. Arterial dilations in response to calcitonin gene-related peptide involve activation of K+ channels. Nature. 1990 Apr 19;344(6268):770–773. doi: 10.1038/344770a0. [DOI] [PubMed] [Google Scholar]
- Nuki C., Kawasaki H., Kitamura K., Takenaga M., Kangawa K., Eto T., Wada A. Vasodilator effect of adrenomedullin and calcitonin gene-related peptide receptors in rat mesenteric vascular beds. Biochem Biophys Res Commun. 1993 Oct 15;196(1):245–251. doi: 10.1006/bbrc.1993.2241. [DOI] [PubMed] [Google Scholar]
- Okumura M., Miura K., Yamashita Y., Yukimura T., Yamamoto K. Role of endothelium-derived relaxing factor in the in vivo renal vascular action of adenosine in dogs. J Pharmacol Exp Ther. 1992 Mar;260(3):1262–1267. [PubMed] [Google Scholar]
- Prieto D., Benedito S., Nyborg N. C. Heterogeneous involvement of endothelium in calcitonin gene-related peptide-induced relaxation in coronary arteries from rat. Br J Pharmacol. 1991 Jul;103(3):1764–1768. doi: 10.1111/j.1476-5381.1991.tb09860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radermacher J., Klanke B., Schurek H. J., Stolte H. F., Frölich J. C. Importance of NO/EDRF for glomerular and tubular function: studies in the isolated perfused rat kidney. Kidney Int. 1992 Jun;41(6):1549–1559. doi: 10.1038/ki.1992.225. [DOI] [PubMed] [Google Scholar]
- Sakata J., Shimokubo T., Kitamura K., Nakamura S., Kangawa K., Matsuo H., Eto T. Molecular cloning and biological activities of rat adrenomedullin, a hypotensive peptide. Biochem Biophys Res Commun. 1993 Sep 15;195(2):921–927. doi: 10.1006/bbrc.1993.2132. [DOI] [PubMed] [Google Scholar]
- Szabó C., Mitchell J. A., Thiemermann C., Vane J. R. Nitric oxide-mediated hyporeactivity to noradrenaline precedes the induction of nitric oxide synthase in endotoxin shock. Br J Pharmacol. 1993 Mar;108(3):786–792. doi: 10.1111/j.1476-5381.1993.tb12879.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALSER M., DAVIDSON D. G., ORLOFF J. The renal clearance of alkali-stable inulin. J Clin Invest. 1955 Oct;34(10):1520–1523. doi: 10.1172/JCI103204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita Y., Yukimura T., Miura K., Okumura M., Yamanaka S., Yamamoto K. Effects of NG-nitro-L-arginine on renal hemodynamic responses to endothelin-3 in anesthetized dogs. J Cardiovasc Pharmacol. 1991;17 (Suppl 7):S332–S334. doi: 10.1097/00005344-199100177-00095. [DOI] [PubMed] [Google Scholar]
- Yukimura T., Yamashita Y., Miura K., Okumura M., Yamanaka S., Yamamoto K. Renal effects of the nitric oxide synthase inhibitor, L-NG-nitroarginine, in dogs. Am J Hypertens. 1992 Jul;5(7):484–487. doi: 10.1093/ajh/5.7.484. [DOI] [PubMed] [Google Scholar]
- Zeidel M. L., Silva P., Brenner B. M., Seifter J. L. cGMP mediates effects of atrial peptides on medullary collecting duct cells. Am J Physiol. 1987 Mar;252(3 Pt 2):F551–F559. doi: 10.1152/ajprenal.1987.252.3.F551. [DOI] [PubMed] [Google Scholar]