Abstract
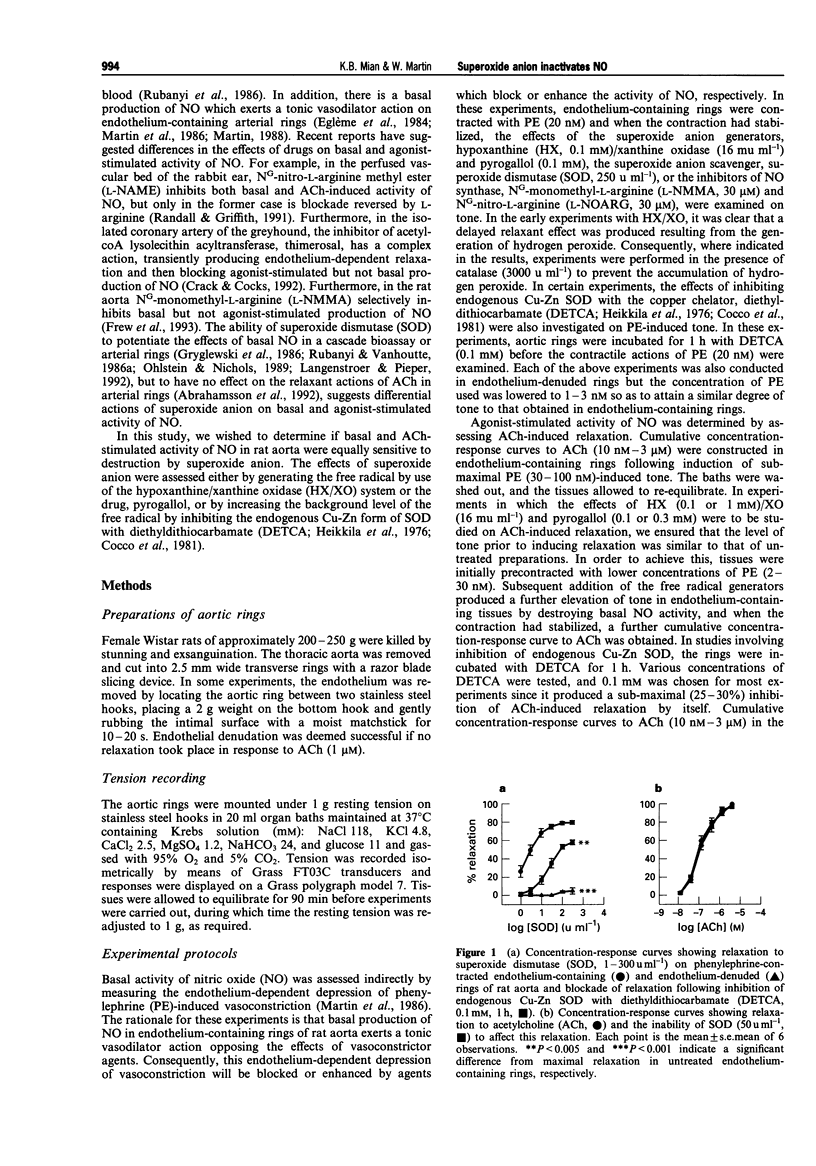
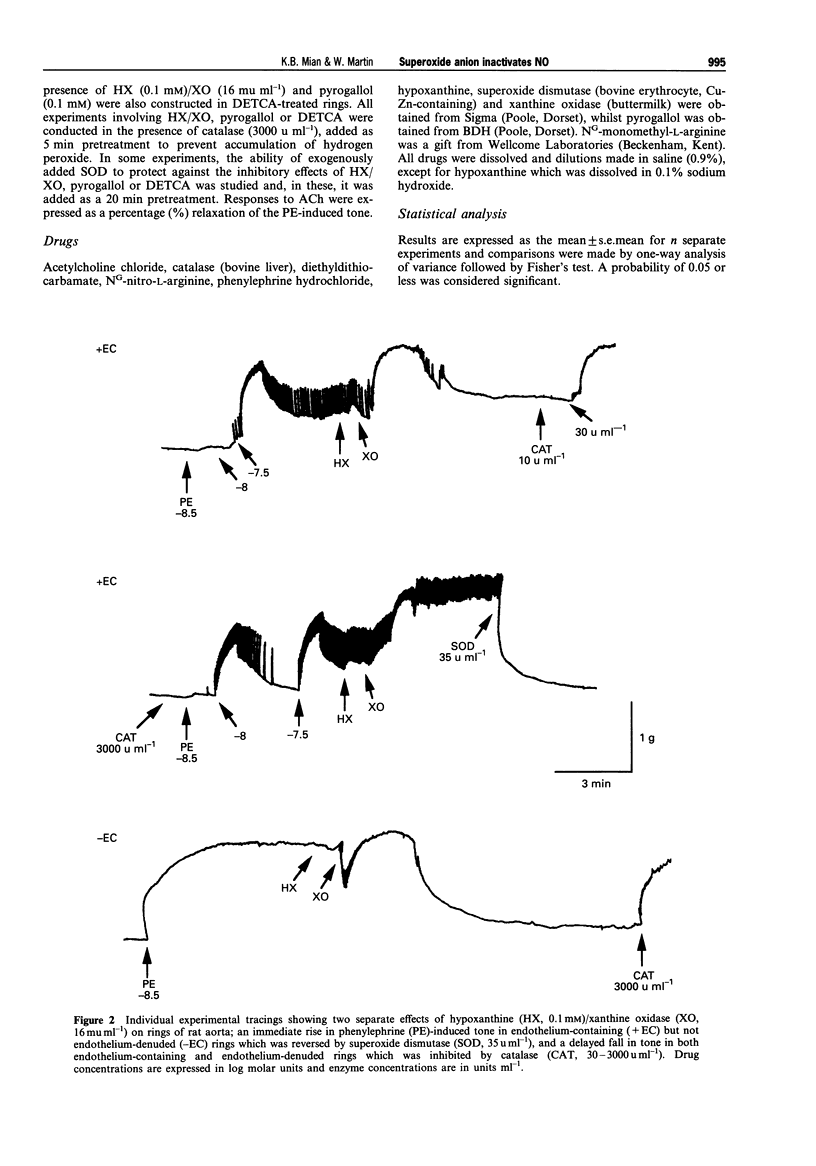
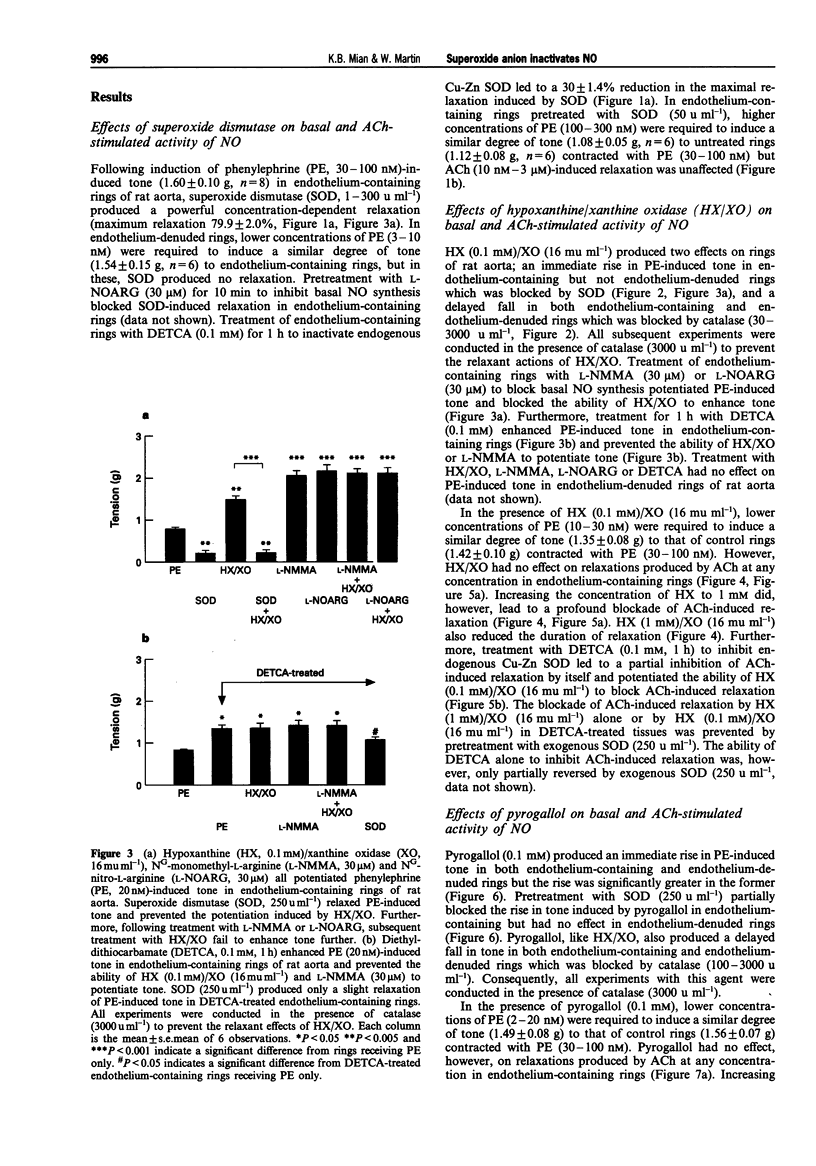
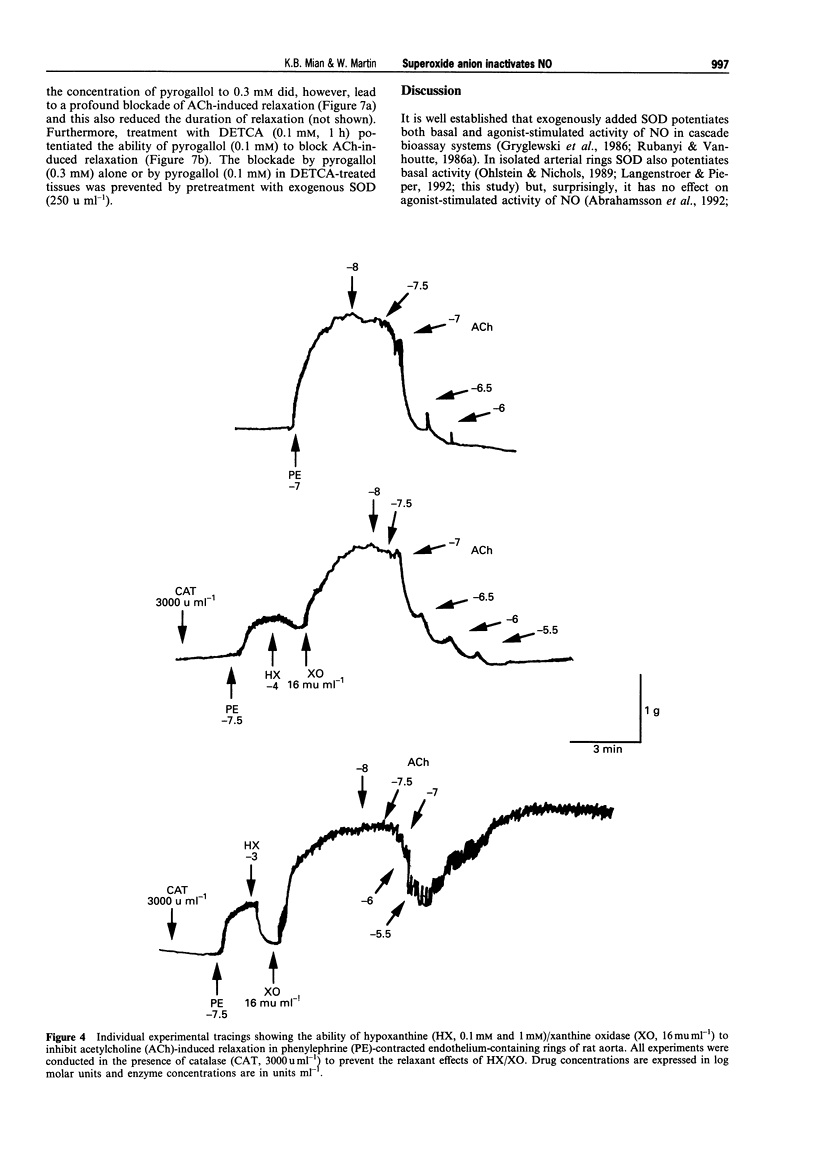
1. In this study we compared the ability of superoxide anion to destroy the relaxant activity of basal and acetylcholine (ACh)-stimulated activity of NO in isolated rings of rat aorta. 2. Superoxide dismutase (SOD, 1-300 u ml-1) induced a concentration-dependent relaxation of phenylephrine (PE)-induced tone in endothelium-containing rings which was blocked by NG-nitro-L-arginine (L-NOARG, 30 microM), but had no effect on endothelium-denuded rings. It was likely therefore that the relaxant action of SOD resulted from protection of basally produced NO from destruction by superoxide anion, generated either within the tissue or in the oxygenated Krebs solution. 3. In contrast, a concentration of SOD (50 u ml-1) which produced almost maximal enhancement of basal NO activity, had no effect on ACh (10 nM-3 microM)-induced relaxation. 4. In the presence of catalase (3000 u ml-1) to prevent the actions of hydrogen peroxide, superoxide anion generation using hypoxanthine (HX, 0.1 mM)/xanthine oxidase (XO, 16 mu ml-1) produced an augmentation of PE-induced tone in endothelium-containing but not endothelium-denuded rings. This was likely to have resulted from removal of the tonic vasodilator action of basally-produced NO by superoxide anion, since it was blocked in tissues treated with SOD (250 u ml-1), NG-monomethyl-L-arginine (L-NMMA, 30 microM) or L-NOARG (30 microM). Pyrogallol (0.1 mM) had a similar action to HX/XO, but produced an additional augmentation of tone by an endothelium-independent mechanism.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrahamsson T., Brandt U., Marklund S. L., Sjöqvist P. O. Vascular bound recombinant extracellular superoxide dismutase type C protects against the detrimental effects of superoxide radicals on endothelium-dependent arterial relaxation. Circ Res. 1992 Feb;70(2):264–271. doi: 10.1161/01.res.70.2.264. [DOI] [PubMed] [Google Scholar]
- Beckman J. S., Beckman T. W., Chen J., Marshall P. A., Freeman B. A. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1620–1624. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke T. M., Wolin M. S. Hydrogen peroxide elicits pulmonary arterial relaxation and guanylate cyclase activation. Am J Physiol. 1987 Apr;252(4 Pt 2):H721–H732. doi: 10.1152/ajpheart.1987.252.4.H721. [DOI] [PubMed] [Google Scholar]
- Cherry P. D., Omar H. A., Farrell K. A., Stuart J. S., Wolin M. S. Superoxide anion inhibits cGMP-associated bovine pulmonary arterial relaxation. Am J Physiol. 1990 Oct;259(4 Pt 2):H1056–H1062. doi: 10.1152/ajpheart.1990.259.4.H1056. [DOI] [PubMed] [Google Scholar]
- Cocco D., Calabrese L., Rigo A., Argese E., Rotilio G. Re-examination of the reaction of diethyldithiocarbamate with the copper of superoxide dismutase. J Biol Chem. 1981 Sep 10;256(17):8983–8986. [PubMed] [Google Scholar]
- Crack P., Cocks T. Thimerosal blocks stimulated but not basal release of endothelium-derived relaxing factor (EDRF) in dog isolated coronary artery. Br J Pharmacol. 1992 Oct;107(2):566–572. doi: 10.1111/j.1476-5381.1992.tb12784.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowell F. J., Hamilton C. A., McMurray J., Reid J. L. Effects of a xanthine oxidase/hypoxanthine free radical and reactive oxygen species generating system on endothelial function in New Zealand white rabbit aortic rings. J Cardiovasc Pharmacol. 1993 Dec;22(6):792–797. doi: 10.1097/00005344-199312000-00003. [DOI] [PubMed] [Google Scholar]
- Downey J. M. Free radicals and their involvement during long-term myocardial ischemia and reperfusion. Annu Rev Physiol. 1990;52:487–504. doi: 10.1146/annurev.ph.52.030190.002415. [DOI] [PubMed] [Google Scholar]
- Eglème C., Godfraind T., Miller R. C. Enhanced responsiveness of rat isolated aorta to clonidine after removal of the endothelial cells. Br J Pharmacol. 1984 Jan;81(1):16–18. doi: 10.1111/j.1476-5381.1984.tb10736.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frew J. D., Paisley K., Martin W. Selective inhibition of basal but not agonist-stimulated activity of nitric oxide in rat aorta by NG-monomethyl-L-arginine. Br J Pharmacol. 1993 Nov;110(3):1003–1008. doi: 10.1111/j.1476-5381.1993.tb13913.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furchgott R. F., Zawadzki J. V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980 Nov 27;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Gryglewski R. J., Palmer R. M., Moncada S. Superoxide anion is involved in the breakdown of endothelium-derived vascular relaxing factor. Nature. 1986 Apr 3;320(6061):454–456. doi: 10.1038/320454a0. [DOI] [PubMed] [Google Scholar]
- Hassan H. M. Biosynthesis and regulation of superoxide dismutases. Free Radic Biol Med. 1988;5(5-6):377–385. doi: 10.1016/0891-5849(88)90111-6. [DOI] [PubMed] [Google Scholar]
- Heikkila R. E., Cabbat F. S., Cohen G. In vivo inhibition of superoxide dismutase in mice by diethyldithiocarbamate. J Biol Chem. 1976 Apr 10;251(7):2182–2185. [PubMed] [Google Scholar]
- Ignarro L. J. Heme-dependent activation of guanylate cyclase by nitric oxide: a novel signal transduction mechanism. Blood Vessels. 1991;28(1-3):67–73. doi: 10.1159/000158845. [DOI] [PubMed] [Google Scholar]
- Katusic Z. S., Vanhoutte P. M. Superoxide anion is an endothelium-derived contracting factor. Am J Physiol. 1989 Jul;257(1 Pt 2):H33–H37. doi: 10.1152/ajpheart.1989.257.1.H33. [DOI] [PubMed] [Google Scholar]
- Kelner M. J., Bagnell R., Hale B., Alexander N. M. Inactivation of intracellular copper-zinc superoxide dismutase by copper chelating agents without glutathione depletion and methemoglobin formation. Free Radic Biol Med. 1989;6(4):355–360. doi: 10.1016/0891-5849(89)90079-8. [DOI] [PubMed] [Google Scholar]
- Kvietys P. R., Inauen W., Bacon B. R., Grisham M. B. Xanthine oxidase-induced injury to endothelium: role of intracellular iron and hydroxyl radical. Am J Physiol. 1989 Nov;257(5 Pt 2):H1640–H1646. doi: 10.1152/ajpheart.1989.257.5.H1640. [DOI] [PubMed] [Google Scholar]
- Langenstroer P., Pieper G. M. Regulation of spontaneous EDRF release in diabetic rat aorta by oxygen free radicals. Am J Physiol. 1992 Jul;263(1 Pt 2):H257–H265. doi: 10.1152/ajpheart.1992.263.1.H257. [DOI] [PubMed] [Google Scholar]
- Lipton S. A., Choi Y. B., Pan Z. H., Lei S. Z., Chen H. S., Sucher N. J., Loscalzo J., Singel D. J., Stamler J. S. A redox-based mechanism for the neuroprotective and neurodestructive effects of nitric oxide and related nitroso-compounds. Nature. 1993 Aug 12;364(6438):626–632. doi: 10.1038/364626a0. [DOI] [PubMed] [Google Scholar]
- Martin W., Furchgott R. F., Villani G. M., Jothianandan D. Depression of contractile responses in rat aorta by spontaneously released endothelium-derived relaxing factor. J Pharmacol Exp Ther. 1986 May;237(2):529–538. [PubMed] [Google Scholar]
- Minor R. L., Jr, Myers P. R., Guerra R., Jr, Bates J. N., Harrison D. G. Diet-induced atherosclerosis increases the release of nitrogen oxides from rabbit aorta. J Clin Invest. 1990 Dec;86(6):2109–2116. doi: 10.1172/JCI114949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore P. K., al-Swayeh O. A., Chong N. W., Evans R. A., Gibson A. L-NG-nitro arginine (L-NOARG), a novel, L-arginine-reversible inhibitor of endothelium-dependent vasodilatation in vitro. Br J Pharmacol. 1990 Feb;99(2):408–412. doi: 10.1111/j.1476-5381.1990.tb14717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers P. R., Guerra R., Jr, Harrison D. G. Release of NO and EDRF from cultured bovine aortic endothelial cells. Am J Physiol. 1989 Apr;256(4 Pt 2):H1030–H1037. doi: 10.1152/ajpheart.1989.256.4.H1030. [DOI] [PubMed] [Google Scholar]
- Myers P. R., Minor R. L., Jr, Guerra R., Jr, Bates J. N., Harrison D. G. Vasorelaxant properties of the endothelium-derived relaxing factor more closely resemble S-nitrosocysteine than nitric oxide. Nature. 1990 May 10;345(6271):161–163. doi: 10.1038/345161a0. [DOI] [PubMed] [Google Scholar]
- Mügge A., Elwell J. H., Peterson T. E., Harrison D. G. Release of intact endothelium-derived relaxing factor depends on endothelial superoxide dismutase activity. Am J Physiol. 1991 Feb;260(2 Pt 1):C219–C225. doi: 10.1152/ajpcell.1991.260.2.C219. [DOI] [PubMed] [Google Scholar]
- Ohlstein E. H., Nichols A. J. Rabbit polymorphonuclear neutrophils elicit endothelium-dependent contraction in vascular smooth muscle. Circ Res. 1989 Oct;65(4):917–924. doi: 10.1161/01.res.65.4.917. [DOI] [PubMed] [Google Scholar]
- Omar H. A., Cherry P. D., Mortelliti M. P., Burke-Wolin T., Wolin M. S. Inhibition of coronary artery superoxide dismutase attenuates endothelium-dependent and -independent nitrovasodilator relaxation. Circ Res. 1991 Sep;69(3):601–608. doi: 10.1161/01.res.69.3.601. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Ferrige A. G., Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987 Jun 11;327(6122):524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Randall M. D., Griffith T. M. Differential effects of L-arginine on the inhibition by NG-nitro-L-arginine methyl ester of basal and agonist-stimulated EDRF activity. Br J Pharmacol. 1991 Nov;104(3):743–749. doi: 10.1111/j.1476-5381.1991.tb12498.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubanyi G. M., Romero J. C., Vanhoutte P. M. Flow-induced release of endothelium-derived relaxing factor. Am J Physiol. 1986 Jun;250(6 Pt 2):H1145–H1149. doi: 10.1152/ajpheart.1986.250.6.H1145. [DOI] [PubMed] [Google Scholar]
- Rubanyi G. M., Vanhoutte P. M. Oxygen-derived free radicals, endothelium, and responsiveness of vascular smooth muscle. Am J Physiol. 1986 May;250(5 Pt 2):H815–H821. doi: 10.1152/ajpheart.1986.250.5.H815. [DOI] [PubMed] [Google Scholar]
- Rubanyi G. M., Vanhoutte P. M. Superoxide anions and hyperoxia inactivate endothelium-derived relaxing factor. Am J Physiol. 1986 May;250(5 Pt 2):H822–H827. doi: 10.1152/ajpheart.1986.250.5.H822. [DOI] [PubMed] [Google Scholar]
- Todoki K., Okabe E., Kiyose T., Sekishita T., Ito H. Oxygen free radical-mediated selective endothelial dysfunction in isolated coronary artery. Am J Physiol. 1992 Mar;262(3 Pt 2):H806–H812. doi: 10.1152/ajpheart.1992.262.3.H806. [DOI] [PubMed] [Google Scholar]
- Wei E. P., Kontos H. A., Christman C. W., DeWitt D. S., Povlishock J. T. Superoxide generation and reversal of acetylcholine-induced cerebral arteriolar dilation after acute hypertension. Circ Res. 1985 Nov;57(5):781–787. doi: 10.1161/01.res.57.5.781. [DOI] [PubMed] [Google Scholar]
- Zembowicz A., Hatchett R. J., Jakubowski A. M., Gryglewski R. J. Involvement of nitric oxide in the endothelium-dependent relaxation induced by hydrogen peroxide in the rabbit aorta. Br J Pharmacol. 1993 Sep;110(1):151–158. doi: 10.1111/j.1476-5381.1993.tb13785.x. [DOI] [PMC free article] [PubMed] [Google Scholar]



