Abstract
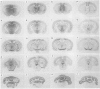
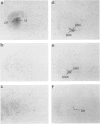
1. Chlorisondamine (CHL), a bisquaternary amine, produces a remarkably long-lasting blockade of central responses to nicotine. The mechanism underlying this blockade is not known. The main aim of this study was to test for possible accumulation of [3H]-CHL in rat brain during the period of chronic blockade. 2. Rats received CHL, either systemically (10 mg kg-1) or centrally (10 micrograms i.c.v.). Seven days later, striatal synaptosomes prepared from these animals were tested for nicotine-induced [3H]-dopamine release. This experiment showed that i.c.v. administration of CHL was as effective as systemic administration in producing ex vivo blockade of central nicotinic receptors. 3. Rats received bilateral i.c.v. infusions of [3H]-CHL (10 micrograms) and radioactivity was subsequently quantified in dissected cerebral cortex, striatum, hippocampus, midbrain and cerebellum. Radiolabel was detected at all three survival times (1, 7, and 21 days). Regional heterogeneity was apparent at 7 and 21 days survival. Radiolabel was almost exclusively confined to the insoluble subcellular fraction in all areas sampled. 4. The anatomical distribution of radiolabel was also visualized in brain sections. Rats received bilateral i.c.v. infusions of [3H]-CHL (10 micrograms) and were killed at 1, 7, 21 or 84 days. Immediately before they were killed, all rats were tested behaviourally, and central nicotinic blockade was demonstrated at 1, 7 and 21 days; partial recovery was observed at 84 days. Particularly at longer survival times, tritium was found to be heavily concentrated in the substantia nigra pars compacta, ventral tegmental area, dorsal raphé nucleus, and the granular layer of the cerebellum. 5. The possibility of retrograde axonal transport of radiolabel was then examined. Rats received a unilateral intrastriatal infusion of [3H]-CHL (0.34 or 0.034 micrograms) one week before they were killed. Autoradiographic labelling was largely confined to the site of infusion and to the ipsilateral substantia nigra pars compacta and dorsal raphé nucleus. 6. Thus, after i.c.v. administration, CHL (and/or centrally-formed derivatives) is initially widely distributed within the brain and is then selectively retained within a few brain areas. A persistent accumulation occurs within putative dopaminergic and 5-hydroxytryptaminergic neurones, at least partly through uptake by terminals and/or axons followed by retrograde transport. This persistent and anatomically-selective intraneuronal accumulation possibly underlies the long-term central nicotinic blockade associated with chlorisondamine.
Full text
PDF

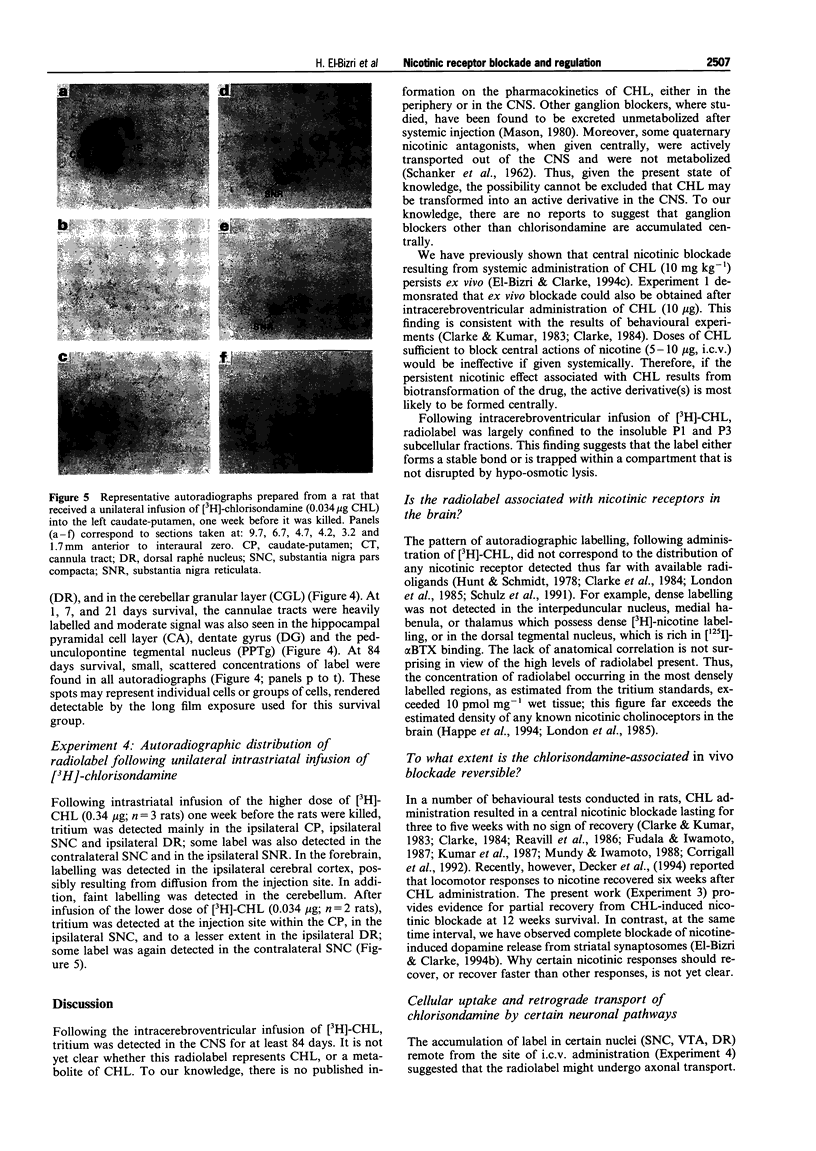


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alkadhi K. A., McIsaac R. J. Effect of preganglionic nerve stimulation on sensitivity of the superior cervical ganglion to nicotinic blocking agents. Br J Pharmacol. 1974 Aug;51(4):533–539. doi: 10.1111/j.1476-5381.1974.tb09671.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avila O. L., Drachman D. B., Pestronk A. Neurotransmission regulates stability of acetylcholine receptors at the neuromuscular junction. J Neurosci. 1989 Aug;9(8):2902–2906. doi: 10.1523/JNEUROSCI.09-08-02902.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Campbell K. J., Takada M., Hattori T. Evidence for retrograde axonal transport of MPP+ in the rat. Neurosci Lett. 1990 Oct 16;118(2):151–154. doi: 10.1016/0304-3940(90)90614-f. [DOI] [PubMed] [Google Scholar]
- Clarke P. B., Chaudieu I., el-Bizri H., Boksa P., Quik M., Esplin B. A., Capek R. The pharmacology of the nicotinic antagonist, chlorisondamine, investigated in rat brain and autonomic ganglion. Br J Pharmacol. 1994 Feb;111(2):397–405. doi: 10.1111/j.1476-5381.1994.tb14748.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke P. B. Chronic central nicotinic blockade after a single administration of the bisquaternary ganglion-blocking drug chlorisondamine. Br J Pharmacol. 1984 Oct;83(2):527–535. doi: 10.1111/j.1476-5381.1984.tb16517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke P. B., Fu D. S., Jakubovic A., Fibiger H. C. Evidence that mesolimbic dopaminergic activation underlies the locomotor stimulant action of nicotine in rats. J Pharmacol Exp Ther. 1988 Aug;246(2):701–708. [PubMed] [Google Scholar]
- Clarke P. B., Kumar R. Characterization of the locomotor stimulant action of nicotine in tolerant rats. Br J Pharmacol. 1983 Nov;80(3):587–594. doi: 10.1111/j.1476-5381.1983.tb10733.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke P. B., Pert C. B., Pert A. Autoradiographic distribution of nicotine receptors in rat brain. Brain Res. 1984 Dec 10;323(2):390–395. doi: 10.1016/0006-8993(84)90320-2. [DOI] [PubMed] [Google Scholar]
- Corrigall W. A., Franklin K. B., Coen K. M., Clarke P. B. The mesolimbic dopaminergic system is implicated in the reinforcing effects of nicotine. Psychopharmacology (Berl) 1992;107(2-3):285–289. doi: 10.1007/BF02245149. [DOI] [PubMed] [Google Scholar]
- Creese R., England J. M. Decamethonium in depolarized muscle and the effects of tubocurarine. J Physiol. 1970 Sep;210(2):345–361. doi: 10.1113/jphysiol.1970.sp009214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creese R., Maclagan J. Entry of decamethonium in rat muscle studied by autoradiography. J Physiol. 1970 Sep;210(2):363–386. doi: 10.1113/jphysiol.1970.sp009215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAHLSTROEM A., FUXE K. A METHOD FOR THE DEMONSTRATION OF MONOAMINE-CONTAINING NERVE FIBRES IN THE CENTRAL NERVOUS SYSTEM. Acta Physiol Scand. 1964 Mar;60:293–294. doi: 10.1111/j.1748-1716.1964.tb02891.x. [DOI] [PubMed] [Google Scholar]
- Donoghue J. P., Herkenham M. Neostriatal projections from individual cortical fields conform to histochemically distinct striatal compartments in the rat. Brain Res. 1986 Feb 19;365(2):397–403. doi: 10.1016/0006-8993(86)91658-6. [DOI] [PubMed] [Google Scholar]
- Fass B., Butcher L. L. Evidence for a crossed nigrostriatal pathway in rats. Neurosci Lett. 1981 Mar 10;22(2):109–113. doi: 10.1016/0304-3940(81)90072-0. [DOI] [PubMed] [Google Scholar]
- Fudala P. J., Iwamoto E. T. Conditioned aversion after delay place conditioning with nicotine. Psychopharmacology (Berl) 1987;92(3):376–381. doi: 10.1007/BF00210847. [DOI] [PubMed] [Google Scholar]
- Fumagalli G., Balbi S., Cangiano A., Lømo T. Regulation of turnover and number of acetylcholine receptors at neuromuscular junctions. Neuron. 1990 Apr;4(4):563–569. doi: 10.1016/0896-6273(90)90114-u. [DOI] [PubMed] [Google Scholar]
- GRIMSON K. S., TARAZI A. K., FRAZER J. W., Jr A new orally active quaternary ammonium, ganglion blocking drug capable of reducing blood pressure, Su-3088. Circulation. 1955 May;11(5):733–741. doi: 10.1161/01.cir.11.5.733. [DOI] [PubMed] [Google Scholar]
- Gerfen C. R., Herkenham M., Thibault J. The neostriatal mosaic: II. Patch- and matrix-directed mesostriatal dopaminergic and non-dopaminergic systems. J Neurosci. 1987 Dec;7(12):3915–3934. doi: 10.1523/JNEUROSCI.07-12-03915.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerfen C. R., Staines W. A., Arbuthnott G. W., Fibiger H. C. Crossed connections of the substantia nigra in the rat. J Comp Neurol. 1982 May 20;207(3):283–303. doi: 10.1002/cne.902070308. [DOI] [PubMed] [Google Scholar]
- Gerfen C. R. The neostriatal mosaic: compartmentalization of corticostriatal input and striatonigral output systems. Nature. 1984 Oct 4;311(5985):461–464. doi: 10.1038/311461a0. [DOI] [PubMed] [Google Scholar]
- Happe H. K., Peters J. L., Bergman D. A., Murrin L. C. Localization of nicotinic cholinergic receptors in rat brain: autoradiographic studies with [3H]cytisine. Neuroscience. 1994 Oct;62(3):929–944. doi: 10.1016/0306-4522(94)90484-7. [DOI] [PubMed] [Google Scholar]
- Herkenham M., Little M. D., Bankiewicz K., Yang S. C., Markey S. P., Johannessen J. N. Selective retention of MPP+ within the monoaminergic systems of the primate brain following MPTP administration: an in vivo autoradiographic study. Neuroscience. 1991;40(1):133–158. doi: 10.1016/0306-4522(91)90180-v. [DOI] [PubMed] [Google Scholar]
- Higgins L. S., Berg D. K. Metabolic stability and antigenic modulation of nicotinic acetylcholine receptors on bovine adrenal chromaffin cells. J Cell Biol. 1988 Sep;107(3):1147–1156. doi: 10.1083/jcb.107.3.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt S., Schmidt J. Some observations on the binding patterns of alpha-bungarotoxin in the central nervous system of the rat. Brain Res. 1978 Nov 24;157(2):213–232. doi: 10.1016/0006-8993(78)90025-2. [DOI] [PubMed] [Google Scholar]
- Kemp G., Edge M. Cholinergic function and alpha-bungarotoxin binding in PC12 cells. Mol Pharmacol. 1987 Sep;32(3):356–363. [PubMed] [Google Scholar]
- Kumar R., Reavill C., Stolerman I. P. Nicotine cue in rats: effects of central administration of ganglion-blocking drugs. Br J Pharmacol. 1987 Jan;90(1):239–246. doi: 10.1111/j.1476-5381.1987.tb16845.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingle C. Blockade of cholinergic channels by chlorisondamine on a crustacean muscle. J Physiol. 1983 Jun;339:395–417. doi: 10.1113/jphysiol.1983.sp014723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingle C. Different types of blockade of crustacean acetylcholine-induced currents. J Physiol. 1983 Jun;339:419–437. doi: 10.1113/jphysiol.1983.sp014724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London E. D., Waller S. B., Wamsley J. K. Autoradiographic localization of [3H]nicotine binding sites in the rat brain. Neurosci Lett. 1985 Jan 21;53(2):179–184. doi: 10.1016/0304-3940(85)90182-x. [DOI] [PubMed] [Google Scholar]
- Maiti A., Shahid Salles K., Grassi S., Abood L. G. Barrel rotation and prostration by vasopressin and nicotine in the vestibular cerebellum. Pharmacol Biochem Behav. 1986 Sep;25(3):583–588. doi: 10.1016/0091-3057(86)90145-0. [DOI] [PubMed] [Google Scholar]
- Moore R. Y., Bloom F. E. Central catecholamine neuron systems: anatomy and physiology of the dopamine systems. Annu Rev Neurosci. 1978;1:129–169. doi: 10.1146/annurev.ne.01.030178.001021. [DOI] [PubMed] [Google Scholar]
- Mundy W. R., Iwamoto E. T. Actions of nicotine on the acquisition of an autoshaped lever-touch response in rats. Psychopharmacology (Berl) 1988;94(2):267–274. doi: 10.1007/BF00176858. [DOI] [PubMed] [Google Scholar]
- Neely A., Lingle C. J. Trapping of an open-channel blocker at the frog neuromuscular acetylcholine channel. Biophys J. 1986 Nov;50(5):981–986. doi: 10.1016/S0006-3495(86)83538-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PLUMMER A. J., TRAPOLD J. H., SCHNEIDER J. A., MAXWELL R. A., EARL A. E. Ganglionic blockade by a new bisquaternary series, including chlorisondamine dimethochloride. J Pharmacol Exp Ther. 1955 Oct;115(2):172–184. [PubMed] [Google Scholar]
- Peng X., Gerzanich V., Anand R., Whiting P. J., Lindstrom J. Nicotine-induced increase in neuronal nicotinic receptors results from a decrease in the rate of receptor turnover. Mol Pharmacol. 1994 Sep;46(3):523–530. [PubMed] [Google Scholar]
- Rapier C., Lunt G. G., Wonnacott S. Stereoselective nicotine-induced release of dopamine from striatal synaptosomes: concentration dependence and repetitive stimulation. J Neurochem. 1988 Apr;50(4):1123–1130. doi: 10.1111/j.1471-4159.1988.tb10582.x. [DOI] [PubMed] [Google Scholar]
- Reavill C., Stolerman I. P., Kumar R., Garcha H. S. Chlorisondamine blocks acquisition of the conditioned taste aversion produced by (-)-nicotine. Neuropharmacology. 1986 Sep;25(9):1067–1069. doi: 10.1016/0028-3908(86)90204-2. [DOI] [PubMed] [Google Scholar]
- Ribak C. E., Vaughn J. E., Roberts E. GABAergic nerve terminals decrease in the substantia nigra following hemitransections of the striatonigral and pallidonigral pathways. Brain Res. 1980 Jun 23;192(2):413–420. doi: 10.1016/0006-8993(80)90893-8. [DOI] [PubMed] [Google Scholar]
- Ribak C. E., Vaughn J. E., Saito K., Barber R., Roberts E. Immunocytochemical localization of glutamate decarboxylase in rat substantia nigra. Brain Res. 1976 Nov 5;116(2):287–298. doi: 10.1016/0006-8993(76)90906-9. [DOI] [PubMed] [Google Scholar]
- SCHNEIDER J. A., MOORE R. F., Jr Electrophysiological investigation of chlorisondamine dimethochloride (Ecolid T.M.) a new ganglionic blocking agent. Proc Soc Exp Biol Med. 1955 Jul;89(3):450–453. doi: 10.3181/00379727-89-21841. [DOI] [PubMed] [Google Scholar]
- Schulz D. W., Loring R. H., Aizenman E., Zigmond R. E. Autoradiographic localization of putative nicotinic receptors in the rat brain using 125I-neuronal bungarotoxin. J Neurosci. 1991 Jan;11(1):287–297. doi: 10.1523/JNEUROSCI.11-01-00287.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbusch H. W., van der Kooy D., Verhofstad A. A., Pellegrino A. Serotonergic and non-serotonergic projections from the nucleus raphe dorsalis to the caudate-putamen complex in the rat, studied by a combined immunofluorescence and fluorescent retrograde axonal labeling technique. Neurosci Lett. 1980 Sep;19(2):137–142. doi: 10.1016/0304-3940(80)90184-6. [DOI] [PubMed] [Google Scholar]
- Traber D. L., Carter V. L., Jr, Gardier R. W. Regarding a necessary condition for ganglionic blockade with competitive agents. Arch Int Pharmacodyn Ther. 1967 Aug;168(2):339–343. [PubMed] [Google Scholar]
- Van Der Kooy D., Hattori T. Single subthalamic nucleus neurons project to both the globus pallidus and substantia nigra in rat. J Comp Neurol. 1980 Aug 15;192(4):751–768. doi: 10.1002/cne.901920409. [DOI] [PubMed] [Google Scholar]
- el-Bizri H., Clarke P. B. Blockade of nicotinic receptor-mediated release of dopamine from striatal synaptosomes by chlorisondamine administered in vivo. Br J Pharmacol. 1994 Feb;111(2):414–418. doi: 10.1111/j.1476-5381.1994.tb14750.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- el-Bizri H., Clarke P. B. Blockade of nicotinic receptor-mediated release of dopamine from striatal synaptosomes by chlorisondamine and other nicotinic antagonists administered in vitro. Br J Pharmacol. 1994 Feb;111(2):406–413. doi: 10.1111/j.1476-5381.1994.tb14749.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Kar L. D., Lorens S. A. Differential serotonergic innervation of individual hypothalamic nuclei and other forebrain regions by the dorsal and median midbrain raphe nuclei. Brain Res. 1979 Feb 16;162(1):45–54. doi: 10.1016/0006-8993(79)90754-6. [DOI] [PubMed] [Google Scholar]