Abstract
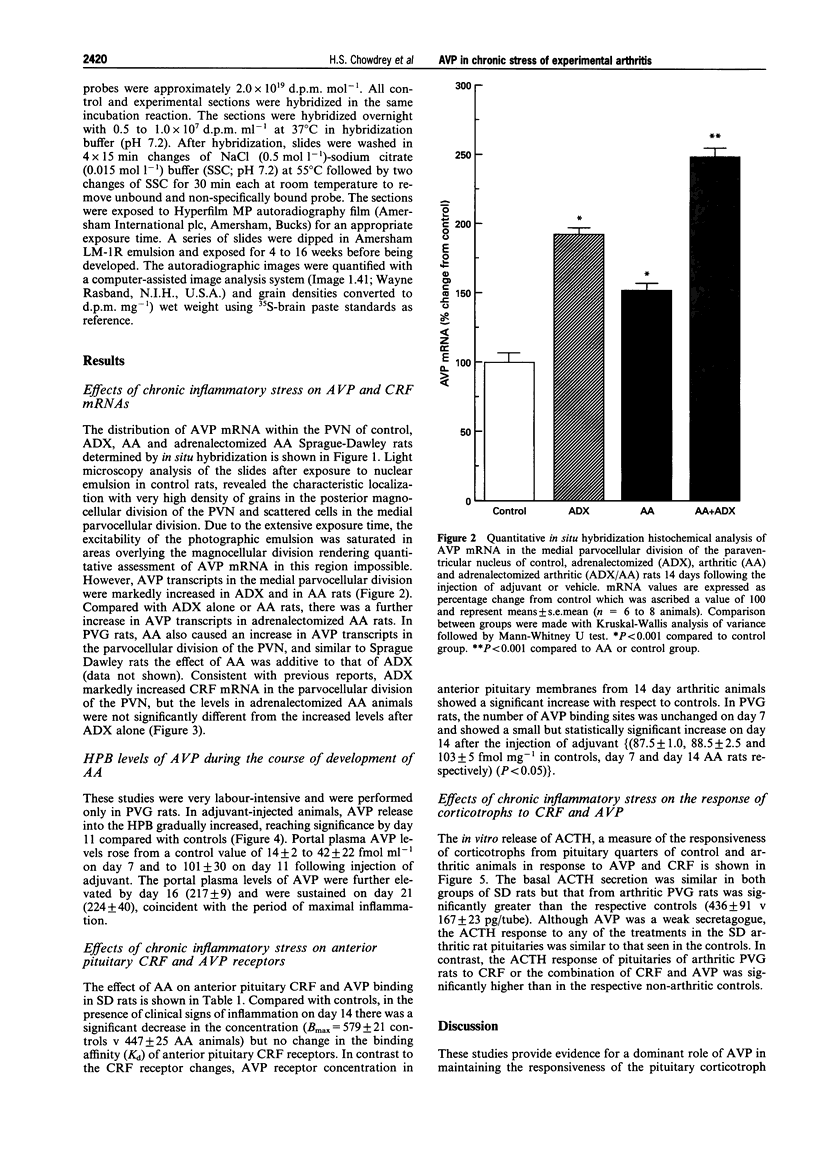
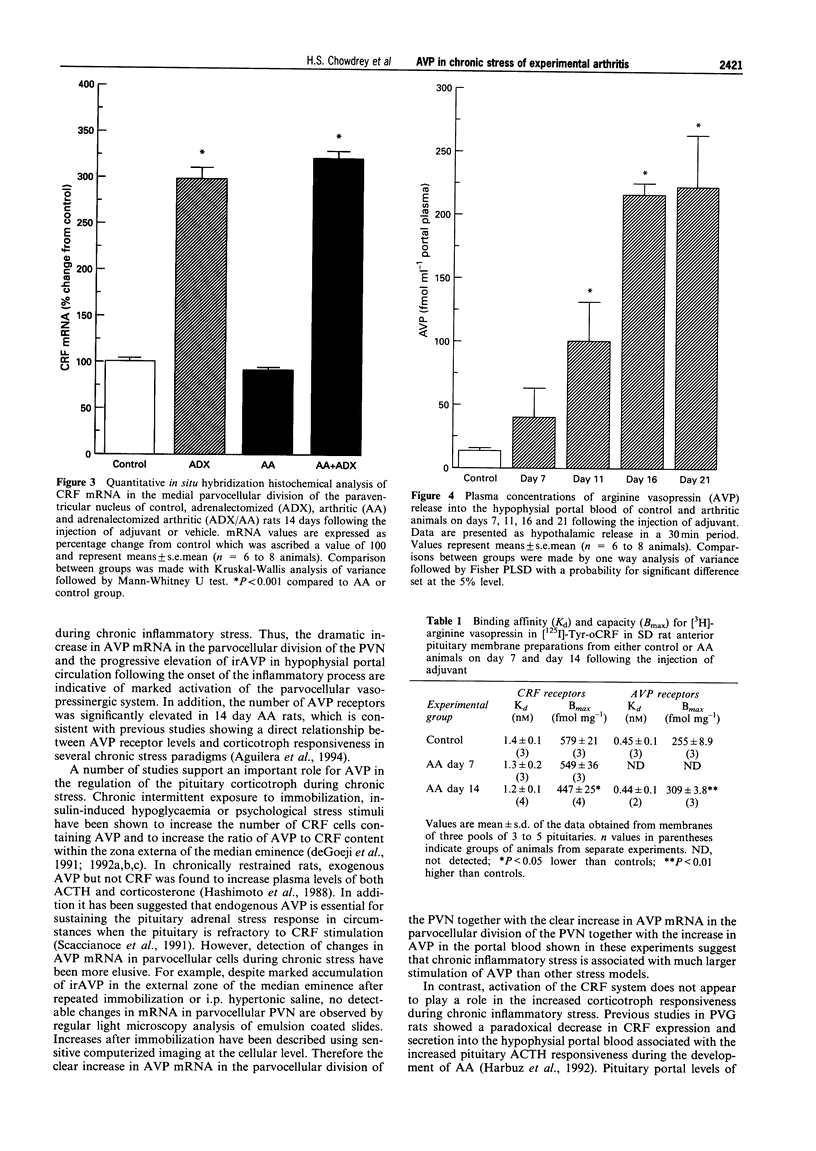
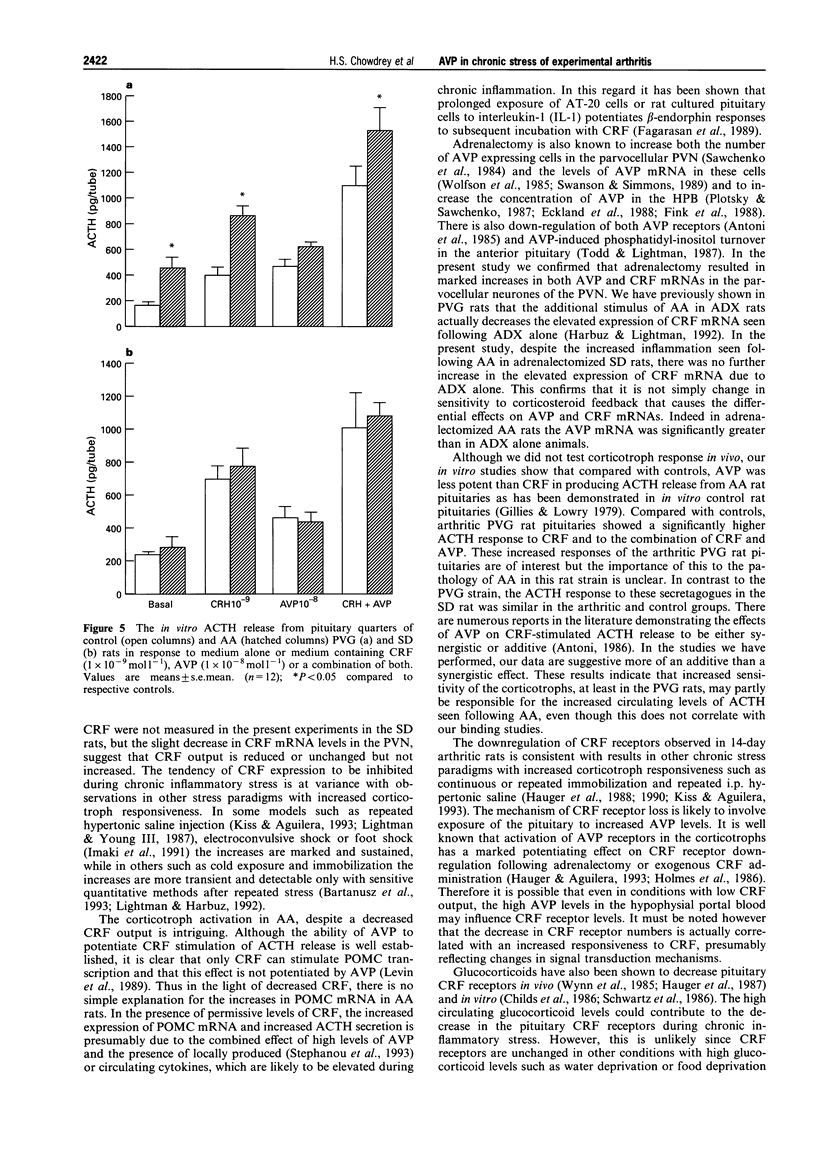
1. Adjuvant-induced arthritis (AA) is an experimental inflammation of the joints that results in chronic activation of the hypothalamo-pituitary-adrenal (HPA) axis. 2. In this study the role of hypothalamic corticotrophin-releasing factor (CRF) and arginine vasopressin (AVP) in the regulation of the HPA axis in this condition both in Sprague-Dawley (SD), and Piebald-Viral-Glaxo (PVG) rats has been further characterized. 3. The increase in AVP peptide content of portal blood (as early as day 11), just prior to the onset of arthritis is confirmed and further increases, peaking at day 16 are shown, coincident with the progression of inflammation in the PVG rats. 4. The increase in AVP is associated with a significant increase in the expression of AVP but not CRF mRNAs in the medial parvocellular division of the hypothalamic paraventricular nucleus (PVN) of arthritic SD rats. 5. In the presence of maximal inflammation of SD rats there was a significant decrease in the maximum binding of [125I]-Tyr-oCRF to anterior pituitary membranes, whereas AVP receptor concentration in anterior pituitary membranes from both PVG and SD rats showed a significant increase with respect to controls. 6. The basal adrenocorticotrophin (ACTH) secretion in vitro was similar in both control and arthritic SD rats but that from arthritic PVG rat pituitaries was significantly greater than the respective controls (436 +/- 91 v 167 +/- 23 pg/tube). The ACTH response of pituitaries of arthritic PVG rats to CRF or the combination of CRF and AVP was significantly higher compared with the controls, although the ACTH response of arthritic SD rat pituitaries was unchanged. 7. The results are consistent with the view that activation of the parvocellular vasopressin system has an important role in the adaptation of the HPA axis to experimentally-induced chronic stress of arthritis.
Full text
PDF



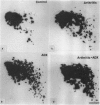
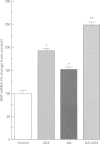
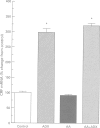
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aguilera G., Pham Q., Rabadan-Diehl C. Regulation of pituitary vasopressin receptors during chronic stress: relationship to corticotroph responsiveness. J Neuroendocrinol. 1994 Jun;6(3):299–304. doi: 10.1111/j.1365-2826.1994.tb00586.x. [DOI] [PubMed] [Google Scholar]
- Antoni F. A., Holmes M. C., Kiss J. Z. Pituitary binding of vasopressin is altered by experimental manipulations of the hypothalamo-pituitary-adrenocortical axis in normal as well as homozygous (di/di) Brattleboro rats. Endocrinology. 1985 Oct;117(4):1293–1299. doi: 10.1210/endo-117-4-1293. [DOI] [PubMed] [Google Scholar]
- Antoni F. A. Hypothalamic control of adrenocorticotropin secretion: advances since the discovery of 41-residue corticotropin-releasing factor. Endocr Rev. 1986 Nov;7(4):351–378. doi: 10.1210/edrv-7-4-351. [DOI] [PubMed] [Google Scholar]
- Bartanusz V., Jezova D., Bertini L. T., Tilders F. J., Aubry J. M., Kiss J. Z. Stress-induced increase in vasopressin and corticotropin-releasing factor expression in hypophysiotrophic paraventricular neurons. Endocrinology. 1993 Feb;132(2):895–902. doi: 10.1210/endo.132.2.8425502. [DOI] [PubMed] [Google Scholar]
- Childs G. V., Morell J. L., Niendorf A., Aguilera G. Cytochemical studies of corticotropin-releasing factor (CRF) receptors in anterior lobe corticotropes: binding, glucocorticoid regulation, and endocytosis of [biotinyl-Ser1]CRF. Endocrinology. 1986 Nov;119(5):2129–2142. doi: 10.1210/endo-119-5-2129. [DOI] [PubMed] [Google Scholar]
- De Goeij D. C., Binnekade R., Tilders F. J. Chronic stress enhances vasopressin but not corticotropin-releasing factor secretion during hypoglycemia. Am J Physiol. 1992 Aug;263(2 Pt 1):E394–E399. doi: 10.1152/ajpendo.1992.263.2.E394. [DOI] [PubMed] [Google Scholar]
- De Goeij D. C., Dijkstra H., Tilders F. J. Chronic psychosocial stress enhances vasopressin, but not corticotropin-releasing factor, in the external zone of the median eminence of male rats: relationship to subordinate status. Endocrinology. 1992 Aug;131(2):847–853. doi: 10.1210/endo.131.2.1322285. [DOI] [PubMed] [Google Scholar]
- Eckland D. J., Todd K., Lightman S. L. Immunoreactive vasopressin and oxytocin in hypothalamo-hypophysial portal blood of the Brattleboro and Long-Evans rat: effect of adrenalectomy and dexamethasone. J Endocrinol. 1988 Apr;117(1):27–34. doi: 10.1677/joe.0.1170027. [DOI] [PubMed] [Google Scholar]
- Fink G., Robinson I. C., Tannahill L. A. Effects of adrenalectomy and glucocorticoids on the peptides CRF-41, AVP and oxytocin in rat hypophysial portal blood. J Physiol. 1988 Jul;401:329–345. doi: 10.1113/jphysiol.1988.sp017165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Făgăraşan M. O., Eskay R., Axelrod J. Interleukin 1 potentiates the secretion of beta-endorphin induced by secretagogues in a mouse pituitary cell line (AtT-20). Proc Natl Acad Sci U S A. 1989 Mar;86(6):2070–2073. doi: 10.1073/pnas.86.6.2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillies G. E., Linton E. A., Lowry P. J. Corticotropin releasing activity of the new CRF is potentiated several times by vasopressin. Nature. 1982 Sep 23;299(5881):355–357. doi: 10.1038/299355a0. [DOI] [PubMed] [Google Scholar]
- Gillies G., Lowry P. Corticotrophin releasing factor may be modulated vasopressin. Nature. 1979 Mar 29;278(5703):463–464. doi: 10.1038/278463a0. [DOI] [PubMed] [Google Scholar]
- Harbuz M. S., Lightman S. L. Responses of hypothalamic and pituitary mRNA to physical and psychological stress in the rat. J Endocrinol. 1989 Sep;122(3):705–711. doi: 10.1677/joe.0.1220705. [DOI] [PubMed] [Google Scholar]
- Harbuz M. S., Lightman S. L. Stress and the hypothalamo-pituitary-adrenal axis: acute, chronic and immunological activation. J Endocrinol. 1992 Sep;134(3):327–339. doi: 10.1677/joe.0.1340327. [DOI] [PubMed] [Google Scholar]
- Harbuz M. S., Rees R. G., Eckland D., Jessop D. S., Brewerton D., Lightman S. L. Paradoxical responses of hypothalamic corticotropin-releasing factor (CRF) messenger ribonucleic acid (mRNA) and CRF-41 peptide and adenohypophysial proopiomelanocortin mRNA during chronic inflammatory stress. Endocrinology. 1992 Mar;130(3):1394–1400. doi: 10.1210/endo.130.3.1537299. [DOI] [PubMed] [Google Scholar]
- Hashimoto K., Suemaru S., Takao T., Sugawara M., Makino S., Ota Z. Corticotropin-releasing hormone and pituitary-adrenocortical responses in chronically stressed rats. Regul Pept. 1988 Nov;23(2):117–126. doi: 10.1016/0167-0115(88)90019-5. [DOI] [PubMed] [Google Scholar]
- Hauger R. L., Aguilera G. Regulation of pituitary corticotropin releasing hormone (CRH) receptors by CRH: interaction with vasopressin. Endocrinology. 1993 Oct;133(4):1708–1714. doi: 10.1210/endo.133.4.8404613. [DOI] [PubMed] [Google Scholar]
- Hauger R. L., Lorang M., Irwin M., Aguilera G. CRF receptor regulation and sensitization of ACTH responses to acute ether stress during chronic intermittent immobilization stress. Brain Res. 1990 Nov 5;532(1-2):34–40. doi: 10.1016/0006-8993(90)91738-3. [DOI] [PubMed] [Google Scholar]
- Hauger R. L., Millan M. A., Catt K. J., Aguilera G. Differential regulation of brain and pituitary corticotropin-releasing factor receptors by corticosterone. Endocrinology. 1987 Apr;120(4):1527–1533. doi: 10.1210/endo-120-4-1527. [DOI] [PubMed] [Google Scholar]
- Hauger R. L., Millan M. A., Lorang M., Harwood J. P., Aguilera G. Corticotropin-releasing factor receptors and pituitary adrenal responses during immobilization stress. Endocrinology. 1988 Jul;123(1):396–405. doi: 10.1210/endo-123-1-396. [DOI] [PubMed] [Google Scholar]
- Herman J. P., Schäfer K. H., Sladek C. D., Day R., Young E. A., Akil H., Watson S. J. Chronic electroconvulsive shock treatment elicits up-regulation of CRF and AVP mRNA in select populations of neuroendocrine neurons. Brain Res. 1989 Nov 6;501(2):235–246. doi: 10.1016/0006-8993(89)90641-0. [DOI] [PubMed] [Google Scholar]
- Holmes M. C., Antoni F. A., Aguilera G., Catt K. J. Magnocellular axons in passage through the median eminence release vasopressin. Nature. 1986 Jan 23;319(6051):326–329. doi: 10.1038/319326a0. [DOI] [PubMed] [Google Scholar]
- Imaki T., Nahan J. L., Rivier C., Sawchenko P. E., Vale W. Differential regulation of corticotropin-releasing factor mRNA in rat brain regions by glucocorticoids and stress. J Neurosci. 1991 Mar;11(3):585–599. doi: 10.1523/JNEUROSCI.11-03-00585.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivell R., Richter D. Structure and comparison of the oxytocin and vasopressin genes from rat. Proc Natl Acad Sci U S A. 1984 Apr;81(7):2006–2010. doi: 10.1073/pnas.81.7.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessop D. S., Eckland D. J., Todd K., Lightman S. L. Osmotic regulation of hypothalamo-neurointermediate lobe corticotrophin-releasing factor-41 in the rat. J Endocrinol. 1989 Jan;120(1):119–124. doi: 10.1677/joe.0.1200119. [DOI] [PubMed] [Google Scholar]
- Jingami H., Matsukura S., Numa S., Imura H. Effects of adrenalectomy and dexamethasone administration on the level of prepro-corticotropin-releasing factor messenger ribonucleic acid (mRNA) in the hypothalamus and adrenocorticotropin/beta-lipotropin precursor mRNA in the pituitary in rats. Endocrinology. 1985 Oct;117(4):1314–1320. doi: 10.1210/endo-117-4-1314. [DOI] [PubMed] [Google Scholar]
- Kiss A., Aguilera G. Regulation of the hypothalamic pituitary adrenal axis during chronic stress: responses to repeated intraperitoneal hypertonic saline injection. Brain Res. 1993 Dec 10;630(1-2):262–270. doi: 10.1016/0006-8993(93)90665-a. [DOI] [PubMed] [Google Scholar]
- Koch B., Lutz-Bucher B. Specific receptors for vasopressin in the pituitary gland: evidence for down-regulation and desensitization to adrenocorticotropin-releasing factors. Endocrinology. 1985 Feb;116(2):671–676. doi: 10.1210/endo-116-2-671. [DOI] [PubMed] [Google Scholar]
- Landgraf R., Szot P., Dorsa D. M. Vasopressin receptors in the brain, liver and kidney of rats following osmotic stimulation. Brain Res. 1991 Mar 29;544(2):287–290. doi: 10.1016/0006-8993(91)90066-5. [DOI] [PubMed] [Google Scholar]
- Levin N., Blum M., Roberts J. L. Modulation of basal and corticotropin-releasing factor-stimulated proopiomelanocortin gene expression by vasopressin in rat anterior pituitary. Endocrinology. 1989 Dec;125(6):2957–2966. doi: 10.1210/endo-125-6-2957. [DOI] [PubMed] [Google Scholar]
- Lightman S. L., Harbuz M. S. Expression of corticotropin-releasing factor mRNA in response to stress. Ciba Found Symp. 1993;172:173–198. doi: 10.1002/9780470514368.ch9. [DOI] [PubMed] [Google Scholar]
- Lightman S. L., Young W. S., 3rd Corticotrophin-releasing factor, vasopressin and pro-opiomelanocortin mRNA responses to stress and opiates in the rat. J Physiol. 1988 Sep;403:511–523. doi: 10.1113/jphysiol.1988.sp017261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lightman S. L., Young W. S., 3rd Influence of steroids on the hypothalamic corticotropin-releasing factor and preproenkephalin mRNA responses to stress. Proc Natl Acad Sci U S A. 1989 Jun;86(11):4306–4310. doi: 10.1073/pnas.86.11.4306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lightman S. L., Young W. S., 3rd Vasopressin, oxytocin, dynorphin, enkephalin and corticotrophin-releasing factor mRNA stimulation in the rat. J Physiol. 1987 Dec;394:23–39. doi: 10.1113/jphysiol.1987.sp016858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munson P. J., Rodbard D. Ligand: a versatile computerized approach for characterization of ligand-binding systems. Anal Biochem. 1980 Sep 1;107(1):220–239. doi: 10.1016/0003-2697(80)90515-1. [DOI] [PubMed] [Google Scholar]
- Nicholson S. A., Adrian T. E., Gillham B., Jones M. T., Bloom S. R. Effect of hypothalamic neuropeptides on corticotrophin release from quarters of rat anterior pituitary gland in vitro. J Endocrinol. 1984 Feb;100(2):219–226. doi: 10.1677/joe.0.1000219. [DOI] [PubMed] [Google Scholar]
- Plotsky P. M., Sawchenko P. E. Hypophysial-portal plasma levels, median eminence content, and immunohistochemical staining of corticotropin-releasing factor, arginine vasopressin, and oxytocin after pharmacological adrenalectomy. Endocrinology. 1987 Apr;120(4):1361–1369. doi: 10.1210/endo-120-4-1361. [DOI] [PubMed] [Google Scholar]
- Rivier C. L., Plotsky P. M. Mediation by corticotropin releasing factor (CRF) of adenohypophysial hormone secretion. Annu Rev Physiol. 1986;48:475–494. doi: 10.1146/annurev.ph.48.030186.002355. [DOI] [PubMed] [Google Scholar]
- Rivier C., Vale W. Interaction of corticotropin-releasing factor and arginine vasopressin on adrenocorticotropin secretion in vivo. Endocrinology. 1983 Sep;113(3):939–942. doi: 10.1210/endo-113-3-939. [DOI] [PubMed] [Google Scholar]
- Rooke P., Baylis P. H. A new sensitive radioimmunoassay for plasma arginine vasopressin. J Immunoassay. 1982;3(2):115–131. doi: 10.1080/15321818208056990. [DOI] [PubMed] [Google Scholar]
- Sarlis N. J., Chowdrey H. S., Stephanou A., Lightman S. L. Chronic activation of the hypothalamo-pituitary-adrenal axis and loss of circadian rhythm during adjuvant-induced arthritis in the rat. Endocrinology. 1992 Apr;130(4):1775–1779. doi: 10.1210/endo.130.4.1312424. [DOI] [PubMed] [Google Scholar]
- Sawchenko P. E. Adrenalectomy-induced enhancement of CRF and vasopressin immunoreactivity in parvocellular neurosecretory neurons: anatomic, peptide, and steroid specificity. J Neurosci. 1987 Apr;7(4):1093–1106. doi: 10.1523/JNEUROSCI.07-04-01093.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawchenko P. E., Swanson L. W., Vale W. W. Co-expression of corticotropin-releasing factor and vasopressin immunoreactivity in parvocellular neurosecretory neurons of the adrenalectomized rat. Proc Natl Acad Sci U S A. 1984 Mar;81(6):1883–1887. doi: 10.1073/pnas.81.6.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaccianoce S., Muscolo L. A., Cigliana G., Navarra D., Nicolai R., Angelucci L. Evidence for a specific role of vasopressin in sustaining pituitary-adrenocortical stress response in the rat. Endocrinology. 1991 Jun;128(6):3138–3143. doi: 10.1210/endo-128-6-3138. [DOI] [PubMed] [Google Scholar]
- Schwartz J., Billestrup N., Perrin M., Rivier J., Vale W. Identification of corticotropin-releasing factor (CRF) target cells and effects of dexamethasone on binding in anterior pituitary using a fluorescent analog of CRF. Endocrinology. 1986 Nov;119(5):2376–2382. doi: 10.1210/endo-119-5-2376. [DOI] [PubMed] [Google Scholar]
- Shewey L. M., Dorsa D. M. Enhanced binding of 3H-arginine8-vasopressin in the Brattleboro rat. Peptides. 1986 Jul-Aug;7(4):701–704. doi: 10.1016/0196-9781(86)90047-1. [DOI] [PubMed] [Google Scholar]
- Stephanou A., Sarlis N. J., Knight R. A., Chowdrey H. S., Lightman S. L. Response of pituitary and spleen pro-opiomelanocortin mRNA, and spleen and thymus interleukin-1 beta mRNA to adjuvant arthritis in the rat. J Neuroimmunol. 1992 Mar;37(1-2):59–63. doi: 10.1016/0165-5728(92)90155-e. [DOI] [PubMed] [Google Scholar]
- Stephanou A., Sarlis N. J., Knight R. A., Lightman S. L., Chowdrey H. S. Effects of cyclosporine A on the hypothalamic-pituitary-adrenal axis and anterior pituitary interleukin-6 mRNA expression during chronic inflammatory stress in the rat. J Neuroimmunol. 1992 Dec;41(2):215–222. doi: 10.1016/0165-5728(92)90072-s. [DOI] [PubMed] [Google Scholar]
- Swanson L. W., Kuypers H. G. The paraventricular nucleus of the hypothalamus: cytoarchitectonic subdivisions and organization of projections to the pituitary, dorsal vagal complex, and spinal cord as demonstrated by retrograde fluorescence double-labeling methods. J Comp Neurol. 1980 Dec 1;194(3):555–570. doi: 10.1002/cne.901940306. [DOI] [PubMed] [Google Scholar]
- Swanson L. W., Simmons D. M. Differential steroid hormone and neural influences on peptide mRNA levels in CRH cells of the paraventricular nucleus: a hybridization histochemical study in the rat. J Comp Neurol. 1989 Jul 22;285(4):413–435. doi: 10.1002/cne.902850402. [DOI] [PubMed] [Google Scholar]
- Todd K., Lightman S. L. Vasopressin activation of phosphatidylinositol metabolism in rat anterior pituitary in vitro and its modification by changes in the hypothalamo-pituitary-adrenal axis. Neuroendocrinology. 1987 Mar;45(3):212–218. doi: 10.1159/000124728. [DOI] [PubMed] [Google Scholar]
- Vale W., Spiess J., Rivier C., Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science. 1981 Sep 18;213(4514):1394–1397. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- Whitnall M. H. Regulation of the hypothalamic corticotropin-releasing hormone neurosecretory system. Prog Neurobiol. 1993 May;40(5):573–629. doi: 10.1016/0301-0082(93)90035-q. [DOI] [PubMed] [Google Scholar]
- Williams T. D., Carter D. A., Lightman S. L. Sexual dimorphism in the posterior pituitary response to stress in the rat. Endocrinology. 1985 Feb;116(2):738–740. doi: 10.1210/endo-116-2-738. [DOI] [PubMed] [Google Scholar]
- Wolfson B., Manning R. W., Davis L. G., Arentzen R., Baldino F., Jr Co-localization of corticotropin releasing factor and vasopressin mRNA in neurones after adrenalectomy. Nature. 1985 May 2;315(6014):59–61. doi: 10.1038/315059a0. [DOI] [PubMed] [Google Scholar]
- Worthington W. C. Blood samples from the pituitary stalk of the rat: method of collection and factors determining volume. Nature. 1966 May 14;210(5037):710–712. doi: 10.1038/210710a0. [DOI] [PubMed] [Google Scholar]
- Wynn P. C., Harwood J. P., Catt K. J., Aguilera G. Regulation of corticotropin-releasing factor (CRF) receptors in the rat pituitary gland: effects of adrenalectomy on CRF receptors and corticotroph responses. Endocrinology. 1985 Apr;116(4):1653–1659. doi: 10.1210/endo-116-4-1653. [DOI] [PubMed] [Google Scholar]
- Young W. S., 3rd, Mezey E., Siegel R. E. Quantitative in situ hybridization histochemistry reveals increased levels of corticotropin-releasing factor mRNA after adrenalectomy in rats. Neurosci Lett. 1986 Oct 8;70(2):198–203. doi: 10.1016/0304-3940(86)90463-5. [DOI] [PubMed] [Google Scholar]
- de Goeij D. C., Jezova D., Tilders F. J. Repeated stress enhances vasopressin synthesis in corticotropin releasing factor neurons in the paraventricular nucleus. Brain Res. 1992 Apr 10;577(1):165–168. doi: 10.1016/0006-8993(92)90552-k. [DOI] [PubMed] [Google Scholar]
- de Goeij D. C., Kvetnansky R., Whitnall M. H., Jezova D., Berkenbosch F., Tilders F. J. Repeated stress-induced activation of corticotropin-releasing factor neurons enhances vasopressin stores and colocalization with corticotropin-releasing factor in the median eminence of rats. Neuroendocrinology. 1991 Feb;53(2):150–159. doi: 10.1159/000125712. [DOI] [PubMed] [Google Scholar]