Abstract
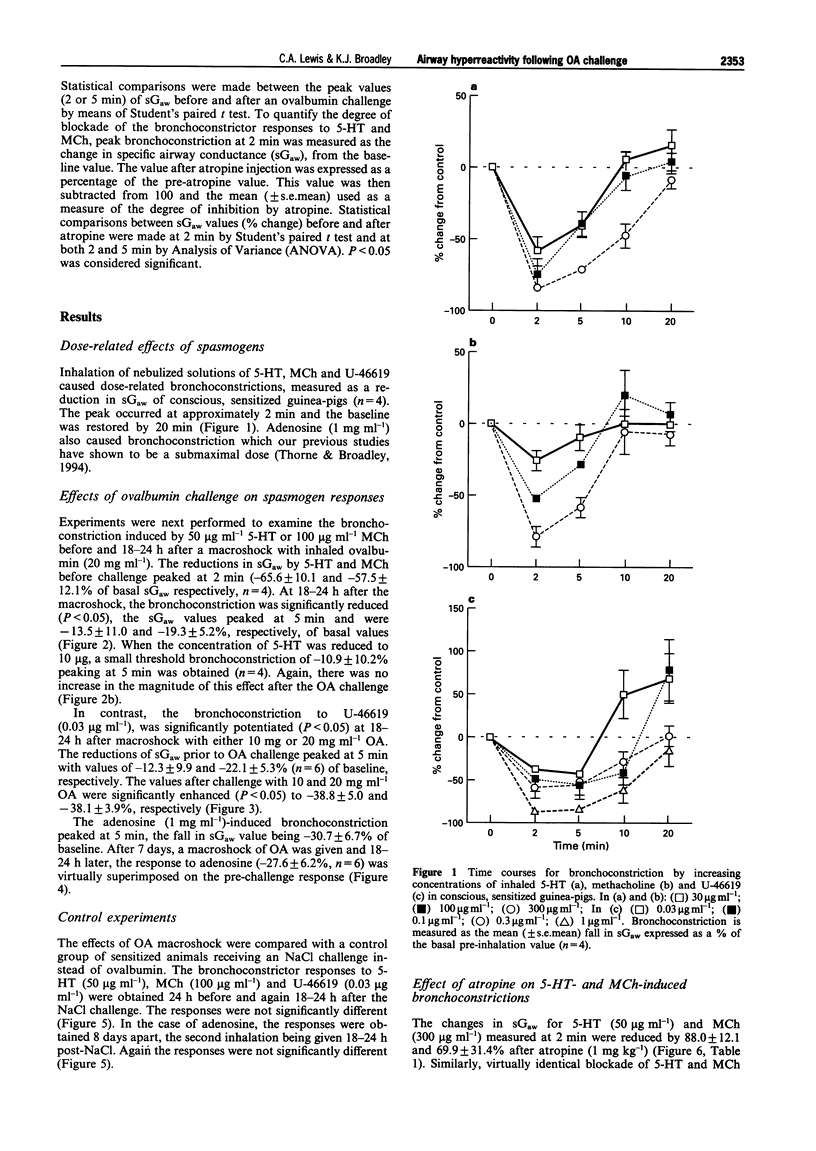
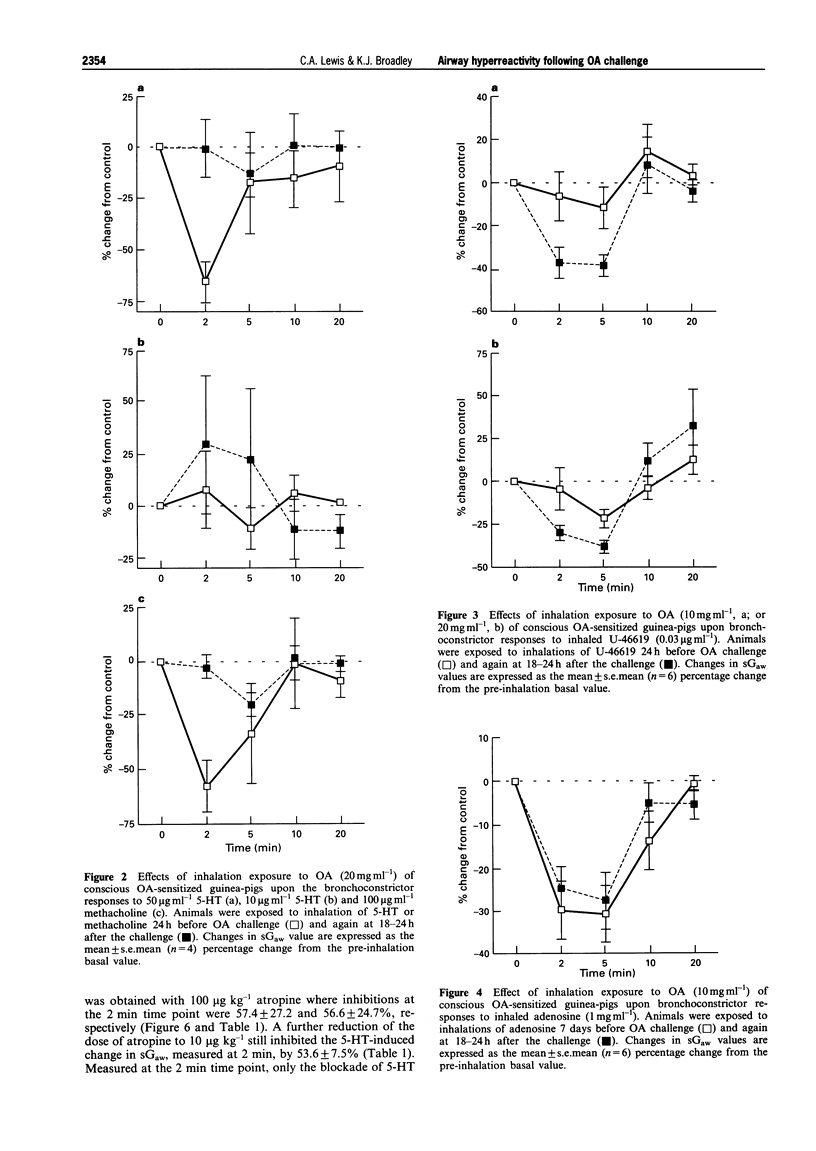
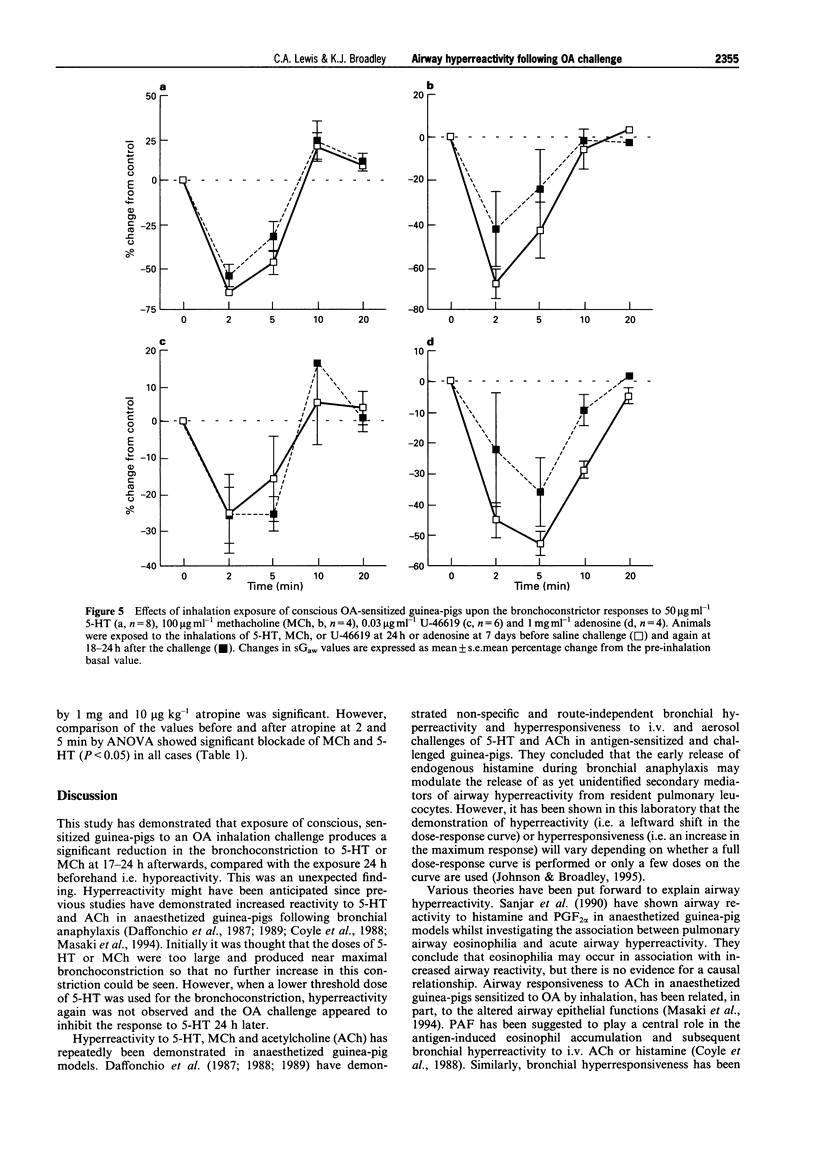
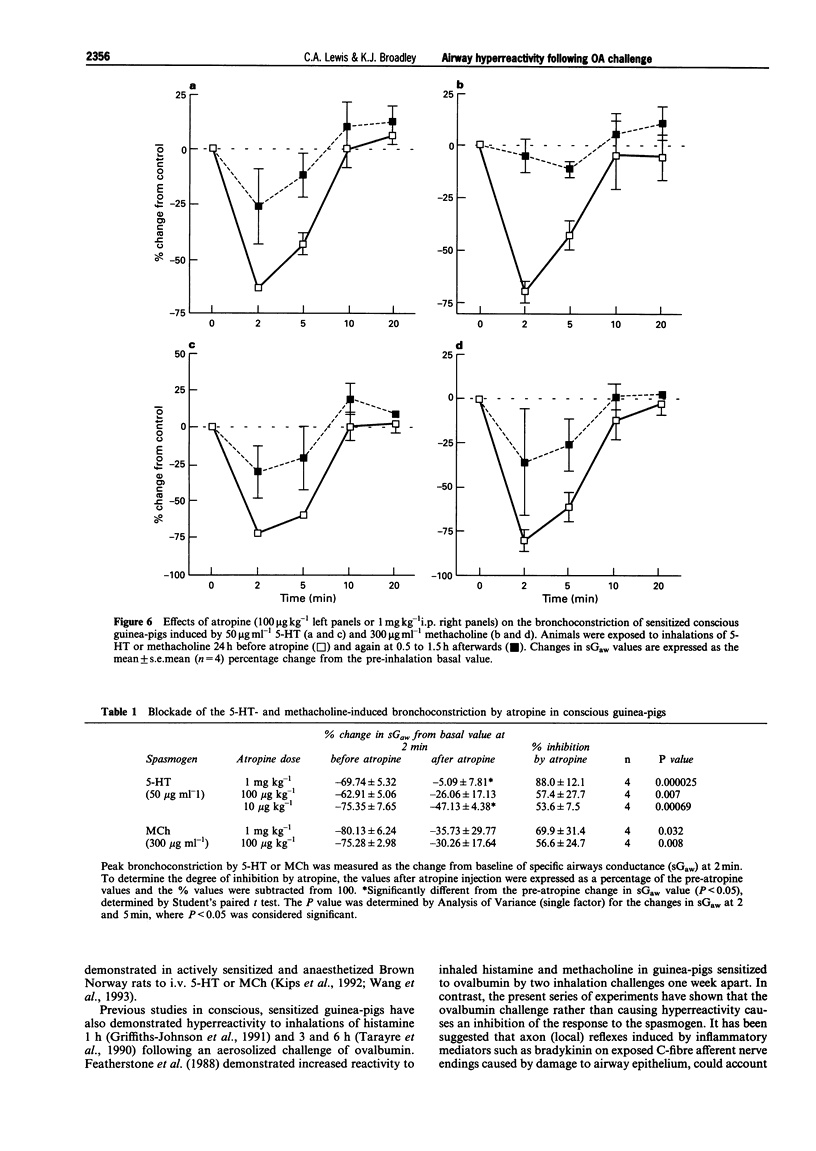
1. The aim of this study was to determine whether an inhalation of ovalbumin (OA, 10 or 20 mg ml-1) by conscious OA-sensitized guinea-pigs leads to airway hyperreactivity to spasmogens 24 h later. In contrast to most previous studies, the spasmogens (5-HT, methacholine (MCh), U-46619 and adenosine) were administered by inhalation and airway function was measured in conscious guinea-pigs. 2. Guinea-pigs were sensitized by i.p. injection of 10 micrograms OA and 100 mg aluminium hydroxide in 1 ml normal saline; 14-21 days later they were exposed to an inhalation of 5-HT, MCh, U-46619 or adenosine. Specific airway conductance (sGaw) was measured in conscious animals by whole body plethysmography. The spasmogens caused bronchoconstriction, measured as a reduction in sGaw from the pre-inhalation basal values. Dose-related bronchoconstrictions were observed with 5-HT, MCh and U-46619. 3. The effect of an ovalbumin macroshock challenge upon the responses to each spasmogen were examined by giving an inhalation of aerosolized OA at 24 h (or 7 days in the cause of adenosine) after an initial spasmogen challenge. Eighteen to twenty-four hours after the OA macroshock, the same guinea-pigs were exposed to a repeated inhalation of 5-HT, MCh, U-46619 or adenosine. 4. U-46619 was the only spasmogen to demonstrate hyperresponsiveness, the peak change in sGaw being increased from -12.3 +/- 9.9 to -38.8 +/- 5.0% by 10 mg ml-1 OA challenge. In contrast, the ovalbumin challenge (20 mg ml-1) inhibited the bronchoconstrictions to 5-HT (50 micrograms ml-1) and MCh (100 micrograms ml-1). Adenosine demonstrated bronchoconstriction in sensitized guinea-pigs but no significant change in the response was observed after an OA challenge. 5. All results were compared with a control group of sensitized guinea-pigs receiving a NaCl challenge. The bronchoconstrictor responses to 5-HT, MCh, U-46619 or adenosine did not differ significantly before and after the saline challenge, indicating reproducibility of the responses. 6. In further experiments, guinea-pigs were exposed to inhalation of 5-HT (50 micrograms ml-1) or MCh (300 micrograms ml-1) 24 h before atropine (10 micrograms, 100 micrograms or 1 mg ml-1) and again at 0.5 to 1.5 h afterwards. Atropine, antagonized the 5-HT- and MCh-induced bronchoconstrictions over the same antagonist dose-range. This suggests that the bronchoconstriction induced in the conscious guinea-pig by 5-HT is mediated primarily via muscarinic receptors, possibly by a vagal reflex. The inhibition of the responses to 5-HT and MCh by OA challenge would therefore appear to be related to interference with a common cholinergic pathway for these spasmogens. 7. In summary, airway hyperresponsiveness was evident at 24 h after OA challenge as measured by an enhanced bronchoconstrictor response to inhaled U-46619. When 5-HT or MCh were used as the spasmogens, an opposing decrease in responsiveness was observed. This was presumed to be due to an inhibition of cholinergic pathways by the OA challenge. Adenosine caused a bronchoconstriction in the sensitized animals but this was not enhanced by the OA challenge.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMDUR M. O., MEAD J. Mechanics of respiration in unanesthetized guinea pigs. Am J Physiol. 1958 Feb;192(2):364–368. doi: 10.1152/ajplegacy.1958.192.2.364. [DOI] [PubMed] [Google Scholar]
- Agrawal K. P. Specific airways conductance in guinea pigs: normal values and histamine induced fall. Respir Physiol. 1981 Jan;43(1):23–30. doi: 10.1016/0034-5687(81)90085-2. [DOI] [PubMed] [Google Scholar]
- Andersson P., Bergstrand H. Antigen-induced bronchial anaphylaxis in actively sensitized guinea-pigs: effect of long-term treatment with sodium cromoglycate and aminophylline. Br J Pharmacol. 1981 Nov;74(3):601–609. doi: 10.1111/j.1476-5381.1981.tb10470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arimura A., Asanuma F., Kurosawa A., Harada M. Contribution of thromboxane A2 to the antigen-induced immediate asthmatic response mediated by IgG1 antibody by augmentation of bronchial responsiveness in guinea-pigs. Br J Pharmacol. 1994 Jan;111(1):339–345. doi: 10.1111/j.1476-5381.1994.tb14065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball D. I., Coleman R. A., Hartley R. W., Newberry A. A novel method for the evaluation of bronchoactive agents in the conscious guinea pig. J Pharmacol Methods. 1991 Nov;26(3):187–202. doi: 10.1016/0160-5402(91)90043-5. [DOI] [PubMed] [Google Scholar]
- Barnes P. J. Asthma as an axon reflex. Lancet. 1986 Feb 1;1(8475):242–245. doi: 10.1016/s0140-6736(86)90777-4. [DOI] [PubMed] [Google Scholar]
- Björck T., Gustafsson L. E., Dahlén S. E. Isolated bronchi from asthmatics are hyperresponsive to adenosine, which apparently acts indirectly by liberation of leukotrienes and histamine. Am Rev Respir Dis. 1992 May;145(5):1087–1091. doi: 10.1164/ajrccm/145.5.1087. [DOI] [PubMed] [Google Scholar]
- Boushey H. A., Holtzman M. J., Sheller J. R., Nadel J. A. Bronchial hyperreactivity. Am Rev Respir Dis. 1980 Feb;121(2):389–413. doi: 10.1164/arrd.1980.121.2.389. [DOI] [PubMed] [Google Scholar]
- Coyle A. J., Urwin S. C., Page C. P., Touvay C., Villain B., Braquet P. The effect of the selective PAF antagonist BN 52021 on PAF- and antigen-induced bronchial hyper-reactivity and eosinophil accumulation. Eur J Pharmacol. 1988 Mar 22;148(1):51–58. doi: 10.1016/0014-2999(88)90453-0. [DOI] [PubMed] [Google Scholar]
- Cushley M. J., Tattersfield A. E., Holgate S. T. Adenosine-induced bronchoconstriction in asthma. Antagonism by inhaled theophylline. Am Rev Respir Dis. 1984 Mar;129(3):380–384. doi: 10.1164/arrd.1984.129.3.380. [DOI] [PubMed] [Google Scholar]
- Daffonchio L., Payne A. N., Lees I. W., Whittle B. J. Airway hyperreactivity follows anaphylactic microshock in anaesthetized guinea-pigs. Eur J Pharmacol. 1989 Feb 28;161(2-3):135–142. doi: 10.1016/0014-2999(89)90835-2. [DOI] [PubMed] [Google Scholar]
- Daffonchio L., Payne A. N., Lees I. W., Whittle B. J. Immediate anaphylactic bronchoconstriction induces airway hyperreactivity in anaesthetized guinea-pigs. Br J Pharmacol. 1988 Jul;94(3):663–668. doi: 10.1111/j.1476-5381.1988.tb11573.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel E. E., O'Byrne P. Effect of inflammatory mediators on airway nerves and muscle. Am Rev Respir Dis. 1991 Mar;143(3 Pt 2):S3–S5. doi: 10.1164/ajrccm/143.3_Pt_2.S3. [DOI] [PubMed] [Google Scholar]
- Durham S. R., Kay A. B. Eosinophils, bronchial hyperreactivity and late-phase asthmatic reactions. Clin Allergy. 1985 Sep;15(5):411–418. doi: 10.1111/j.1365-2222.1985.tb02290.x. [DOI] [PubMed] [Google Scholar]
- Featherstone R. L., Hutson P. A., Holgate S. T., Church M. K. Active sensitization of guinea-pig airways in vivo enhances in vivo and in vitro responsiveness. Eur Respir J. 1988 Oct;1(9):839–845. [PubMed] [Google Scholar]
- Griffiths-Johnson D. A., Karol M. H. Validation of a non-invasive technique to assess development of airway hyperreactivity in an animal model of immunologic pulmonary hypersensitivity. Toxicology. 1991 Jan;65(3):283–294. doi: 10.1016/0300-483x(91)90087-h. [DOI] [PubMed] [Google Scholar]
- Griffiths-Johnson D. A., Nicholls P. J., McDermott M. Measurement of specific airway conductance in guinea pigs. A noninvasive method. J Pharmacol Methods. 1988 May;19(3):233–242. doi: 10.1016/0160-5402(88)90025-3. [DOI] [PubMed] [Google Scholar]
- Hahn H. L., Wilson A. G., Graf P. D., Fischer S. P., Nadel J. A. Interaction between serotonin and efferent vagus nerves in dog lungs. J Appl Physiol Respir Environ Exerc Physiol. 1978 Feb;44(2):144–149. doi: 10.1152/jappl.1978.44.2.144. [DOI] [PubMed] [Google Scholar]
- Hsiue T. R., Leff A. R., Garland A., Hershenson M. B., Ray D. W., Solway J. Impaired sensorineural function after allergen-induced mediator release. Am Rev Respir Dis. 1993 Aug;148(2):447–454. doi: 10.1164/ajrccm/148.2.447. [DOI] [PubMed] [Google Scholar]
- Ishida K., Kelly L. J., Thomson R. J., Beattie L. L., Schellenberg R. R. Repeated antigen challenge induces airway hyperresponsiveness with tissue eosinophilia in guinea pigs. J Appl Physiol (1985) 1989 Sep;67(3):1133–1139. doi: 10.1152/jappl.1989.67.3.1133. [DOI] [PubMed] [Google Scholar]
- Jones G. L., Saroea H. G., Watson R. M., O'Byrne P. M. Effect of an inhaled thromboxane mimetic (U46619) on airway function in human subjects. Am Rev Respir Dis. 1992 Jun;145(6):1270–1274. doi: 10.1164/ajrccm/145.6.1270. [DOI] [PubMed] [Google Scholar]
- Kips J. C., Cuvelier C. A., Pauwels R. A. Effect of acute and chronic antigen inhalation on airway morphology and responsiveness in actively sensitized rats. Am Rev Respir Dis. 1992 Jun;145(6):1306–1310. doi: 10.1164/ajrccm/145.6.1306. [DOI] [PubMed] [Google Scholar]
- Mann J. S., Holgate S. T., Renwick A. G., Cushley M. J. Airway effects of purine nucleosides and nucleotides and release with bronchial provocation in asthma. J Appl Physiol (1985) 1986 Nov;61(5):1667–1676. doi: 10.1152/jappl.1986.61.5.1667. [DOI] [PubMed] [Google Scholar]
- Masaki Y., Munakata M., Amishima M., Homma Y., Kawakami Y. In vivo, in vitro correlation of acetylcholine airway responsiveness in sensitized guinea pigs. The role of modified epithelial functions. Am J Respir Crit Care Med. 1994 Jun;149(6):1494–1498. doi: 10.1164/ajrccm.149.6.8004304. [DOI] [PubMed] [Google Scholar]
- Matsuse T., Thomson R. J., Chen X. R., Salari H., Schellenberg R. R. Capsaicin inhibits airway hyperresponsiveness but not lipoxygenase activity or eosinophilia after repeated aerosolized antigen in guinea pigs. Am Rev Respir Dis. 1991 Aug;144(2):368–372. doi: 10.1164/ajrccm/144.2.368. [DOI] [PubMed] [Google Scholar]
- Payne A. N., de Nucci G. Anaphylaxis in guinea pigs induced by ovalbumin aerosol in vivo and in vitro methods. J Pharmacol Methods. 1987 Mar;17(1):83–90. doi: 10.1016/0160-5402(87)90040-4. [DOI] [PubMed] [Google Scholar]
- Phillips G. D., Bagga P. K., Djukanovic R., Holgate S. T. The influence of refractoriness to adenosine 5'-monophosphate on allergen-provoked bronchoconstriction in asthma. Am Rev Respir Dis. 1989 Aug;140(2):321–326. doi: 10.1164/ajrccm/140.2.321. [DOI] [PubMed] [Google Scholar]
- Raeburn D., Underwood S. L., Villamil M. E. Techniques for drug delivery to the airways, and the assessment of lung function in animal models. J Pharmacol Toxicol Methods. 1992 May;27(3):143–159. doi: 10.1016/1056-8719(92)90035-y. [DOI] [PubMed] [Google Scholar]
- Sanjar S., Aoki S., Kristersson A., Smith D., Morley J. Antigen challenge induces pulmonary airway eosinophil accumulation and airway hyperreactivity in sensitized guinea-pigs: the effect of anti-asthma drugs. Br J Pharmacol. 1990 Apr;99(4):679–686. doi: 10.1111/j.1476-5381.1990.tb12989.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarayre J. P., Aliaga M., Barbara M., Tisseyre N., Vieu S., Tisne-Versailles J. Model of bronchial hyperreactivity after active anaphylactic shock in conscious guinea pigs. J Pharmacol Methods. 1990 Mar;23(1):13–19. doi: 10.1016/0160-5402(90)90004-5. [DOI] [PubMed] [Google Scholar]
- Taylor S. M., Paré P. D., Armour C. L., Hogg J. C., Schellenberg R. R. Airway reactivity in chronic obstructive pulmonary disease. Failure of in vivo methacholine responsiveness to correlate with cholinergic, adrenergic, or nonadrenergic responses in vitro. Am Rev Respir Dis. 1985 Jul;132(1):30–35. doi: 10.1164/arrd.1985.132.1.30. [DOI] [PubMed] [Google Scholar]
- Thorne J. R., Broadley K. J. Adenosine-induced bronchoconstriction in conscious hyperresponsive and sensitized guinea pigs. Am J Respir Crit Care Med. 1994 Feb;149(2 Pt 1):392–399. doi: 10.1164/ajrccm.149.2.8306036. [DOI] [PubMed] [Google Scholar]
- Thorne J. R., Broadley K. J. Adenosine-induced bronchoconstriction of isolated lung and trachea from sensitized guinea-pigs. Br J Pharmacol. 1992 Aug;106(4):978–985. doi: 10.1111/j.1476-5381.1992.tb14445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C. G., Du T., Xu L. J., Martin J. G. Role of leukotriene D4 in allergen-induced increases in airway smooth muscle in the rat. Am Rev Respir Dis. 1993 Aug;148(2):413–417. doi: 10.1164/ajrccm/148.2.413. [DOI] [PubMed] [Google Scholar]



