Abstract
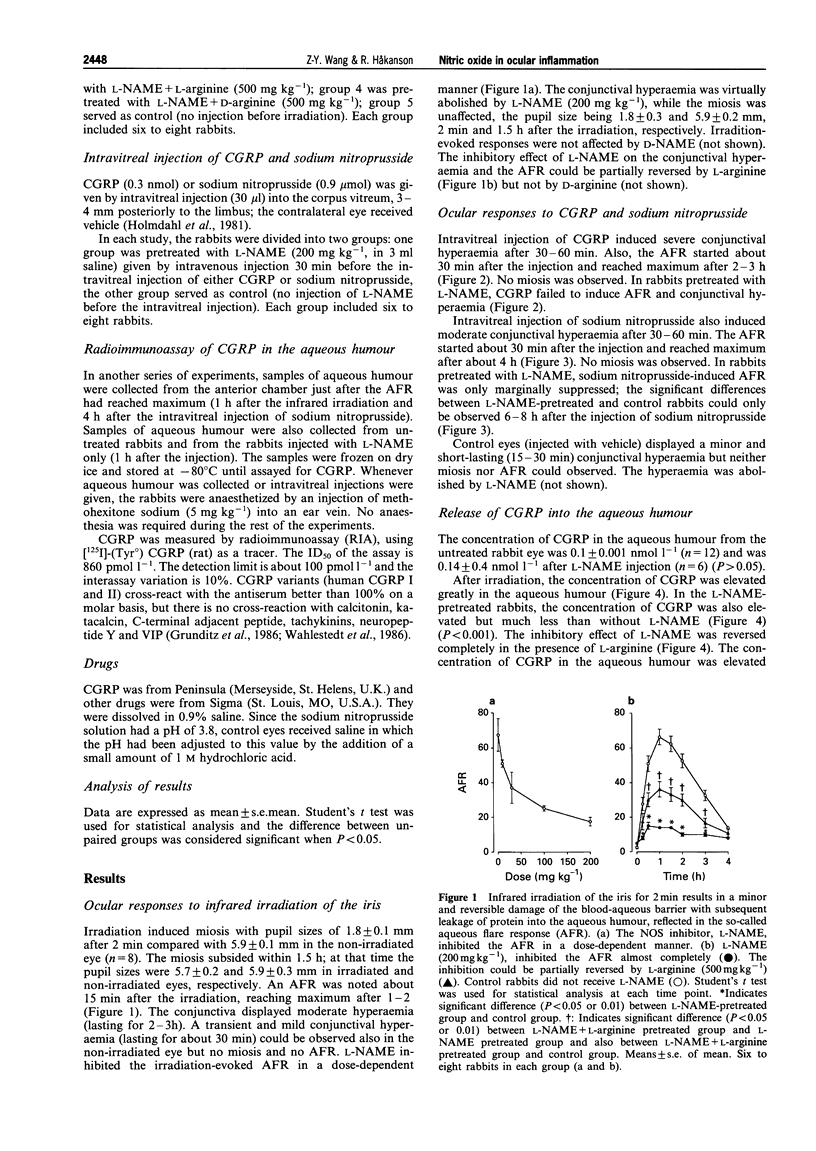
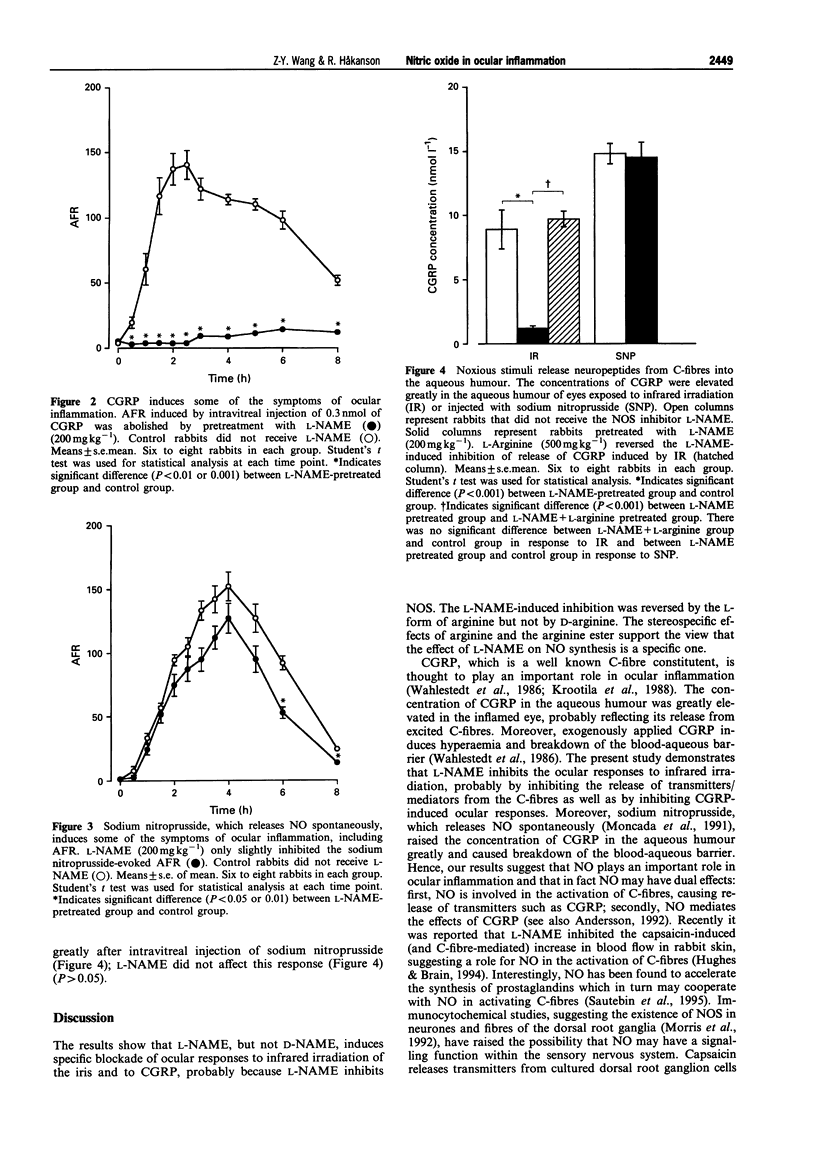
1. The actions of nitric oxide (NO) have been investigated in the rabbit eye, with particular emphasis on the relationship between NO and C-fibres and on those effects of NO that may be of importance in the inflammatory response to C-fibre stimulation. 2. The NO synthase inhibitor, NG-nitro-L-arginine (L-NAME; 10-200 mg kg-1), but not the inactive analogue D-NAME (200 mg kg-1), was found to block the inflammatory response induced by infrared irradiation of the iris in a dose-dependent manner. The inhibitory effects of L-NAME (200 mg kg-1) were partially reversed by L-arginine (500 mg kg-1), but not by D-arginine (500 mg kg-1). 3. L-NAME (200 mg kg-1) virtually abolished the ocular effects of intravitreal injection of calcitonin gene-related peptide (CGRP) (0.3 nmol). 4. The concentration of CGRP in aqueous humour from untreated rabbit eyes was 0.1 +/- 0.001 nmol l-1. Irradiation of the iris raised the CGRP concentration to 8.9 +/- 1.5 nmol l-1. L-NAME (200 mg kg-1) greatly suppressed the irradiation-evoked release of CGRP, the concentration in the aqueous humour being 1.2 +/- 0.2 nmol l-1 (P < 0.001). L-Arginine reversed the L-NAME-induced inhibition of release of CGRP, the concentration of CGRP in the aqueous humour being 9.7 +/- 0.6 nmol l-1. 5. In addition, a NO donor, sodium nitroprusside (0.9 mumol), was found to raise the concentration of CGRP in the aqueous humour (14.8 +/- 0.8 nmol l-1) and to induce symptoms of ocular inflammation. The elevation in concentration of CGRP induced by sodium nitroprusside was not affected by L-NAME (200 mg kg-1) (14.5 +/- 1.2 nmol l-1). Ocular responses were not inhibited by L-NAME. 6. Our findings suggest that NO plays an important role in ocular inflammation by activating C-fibres (directly or indirectly) and by mediating CGRP-induced responses.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANJOU C. I., KRAKAU C. E. Aqueous flare and protein content in the anterior chamber of normal rabbits' eyes. Acta Ophthalmol (Copenh) 1961;39:95–101. doi: 10.1111/j.1755-3768.1961.tb00244.x. [DOI] [PubMed] [Google Scholar]
- Andersson S. E. Glibenclamide and L-NG-nitro-arginine methyl ester modulate the ocular and hypotensive effects of calcitonin gene-related peptide. Eur J Pharmacol. 1992 Nov 24;224(1):89–91. doi: 10.1016/0014-2999(92)94823-e. [DOI] [PubMed] [Google Scholar]
- DYSTER-AAS K., KRAKAU C. E. AQUEOUS FLOW DETERMINATION IN THE RABBIT BY MEANS OF A MINIMAL EYE TRAUMA. Invest Ophthalmol. 1964 Apr;3:127–134. [PubMed] [Google Scholar]
- Grider J. R., Murthy K. S., Jin J. G., Makhlouf G. M. Stimulation of nitric oxide from muscle cells by VIP: prejunctional enhancement of VIP release. Am J Physiol. 1992 Apr;262(4 Pt 1):G774–G778. doi: 10.1152/ajpgi.1992.262.4.G774. [DOI] [PubMed] [Google Scholar]
- Grunditz T., Ekman R., Håkanson R., Rerup C., Sundler F., Uddman R. Calcitonin gene-related peptide in thyroid nerve fibers and C cells: effects on thyroid hormone secretion and response to hypercalcemia. Endocrinology. 1986 Nov;119(5):2313–2324. doi: 10.1210/endo-119-5-2313. [DOI] [PubMed] [Google Scholar]
- Holmdahl G., Håkanson R., Leander S., Rosell S., Folkers K., Sundler F. A substance P antagonist, [D-Pro2, D-Trp7,9]SP, inhibits inflammatory responses in the rabbit eye. Science. 1981 Nov 27;214(4524):1029–1031. doi: 10.1126/science.6171036. [DOI] [PubMed] [Google Scholar]
- Holzer P. Capsaicin: cellular targets, mechanisms of action, and selectivity for thin sensory neurons. Pharmacol Rev. 1991 Jun;43(2):143–201. [PubMed] [Google Scholar]
- Hughes S. R., Brain S. D. Nitric oxide-dependent release of vasodilator quantities of calcitonin gene-related peptide from capsaicin-sensitive nerves in rabbit skin. Br J Pharmacol. 1994 Feb;111(2):425–430. doi: 10.1111/j.1476-5381.1994.tb14752.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krootila K., Uusitalo H., Palkama A. Effect of neurogenic irritation and calcitonin gene-related peptide (CGRP) on ocular blood flow in the rabbit. Curr Eye Res. 1988 Jul;7(7):695–703. doi: 10.3109/02713688809033199. [DOI] [PubMed] [Google Scholar]
- Moncada S., Palmer R. M., Higgs E. A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991 Jun;43(2):109–142. [PubMed] [Google Scholar]
- Morris R., Southam E., Braid D. J., Garthwaite J. Nitric oxide may act as a messenger between dorsal root ganglion neurones and their satellite cells. Neurosci Lett. 1992 Mar 16;137(1):29–32. doi: 10.1016/0304-3940(92)90290-n. [DOI] [PubMed] [Google Scholar]
- Osborne N. N., Barnett N. L., Herrera A. J. NADPH diaphorase localization and nitric oxide synthetase activity in the retina and anterior uvea of the rabbit eye. Brain Res. 1993 May 7;610(2):194–198. doi: 10.1016/0006-8993(93)91400-m. [DOI] [PubMed] [Google Scholar]
- Persson M. G., Wiklund N. P., Gustafsson L. E. Nitric oxide requirement for vasomotor nerve-induced vasodilatation and modulation of resting blood flow in muscle microcirculation. Acta Physiol Scand. 1991 Jan;141(1):49–56. doi: 10.1111/j.1748-1716.1991.tb09043.x. [DOI] [PubMed] [Google Scholar]
- Sanders K. M., Ward S. M. Nitric oxide as a mediator of nonadrenergic noncholinergic neurotransmission. Am J Physiol. 1992 Mar;262(3 Pt 1):G379–G392. doi: 10.1152/ajpgi.1992.262.3.G379. [DOI] [PubMed] [Google Scholar]
- Sautebin L., Ialenti A., Ianaro A., Di Rosa M. Modulation by nitric oxide of prostaglandin biosynthesis in the rat. Br J Pharmacol. 1995 Jan;114(2):323–328. doi: 10.1111/j.1476-5381.1995.tb13230.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seligsohn E. E., Bill A. Effects of NG-nitro-L-arginine methyl ester on the cardiovascular system of the anaesthetized rabbit and on the cardiovascular response to thyrotropin-releasing hormone. Br J Pharmacol. 1993 Aug;109(4):1219–1225. doi: 10.1111/j.1476-5381.1993.tb13752.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder S. H., Bredt D. S. Nitric oxide as a neuronal messenger. Trends Pharmacol Sci. 1991 Apr;12(4):125–128. doi: 10.1016/0165-6147(91)90526-x. [DOI] [PubMed] [Google Scholar]
- Stone R. A., Kuwayama Y., Laties A. M. Regulatory peptides in the eye. Experientia. 1987 Jul 15;43(7):791–800. doi: 10.1007/BF01945357. [DOI] [PubMed] [Google Scholar]
- Unger W. G. Review: mediation of the ocular response to injury. J Ocul Pharmacol. 1990 Winter;6(4):337–353. doi: 10.1089/jop.1990.6.337. [DOI] [PubMed] [Google Scholar]
- Wahlestedt C., Beding B., Ekman R., Oksala O., Stjernschantz J., Håkanson R. Calcitonin gene-related peptide in the eye: release by sensory nerve stimulation and effects associated with neurogenic inflammation. Regul Pept. 1986 Dec 22;16(2):107–115. doi: 10.1016/0167-0115(86)90054-6. [DOI] [PubMed] [Google Scholar]
- Yamamoto R., Bredt D. S., Snyder S. H., Stone R. A. The localization of nitric oxide synthase in the rat eye and related cranial ganglia. Neuroscience. 1993 May;54(1):189–200. doi: 10.1016/0306-4522(93)90393-t. [DOI] [PubMed] [Google Scholar]


