Abstract
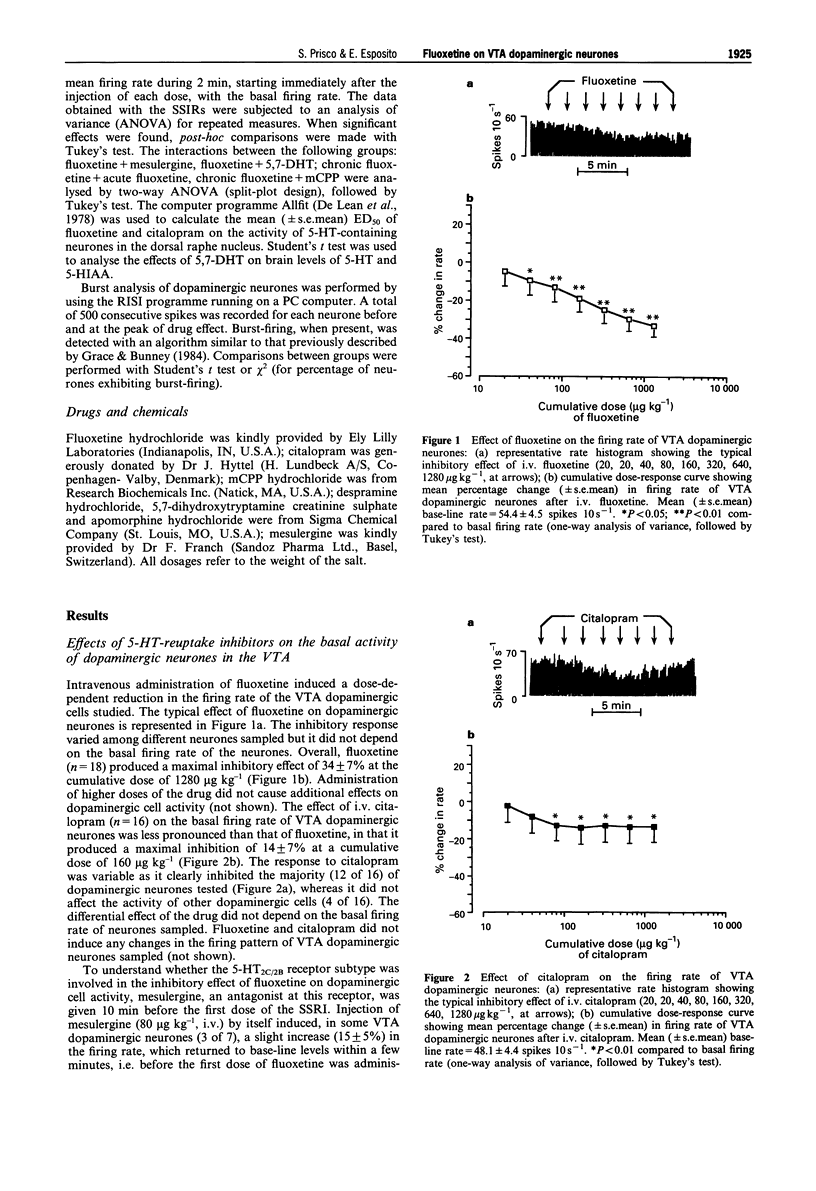
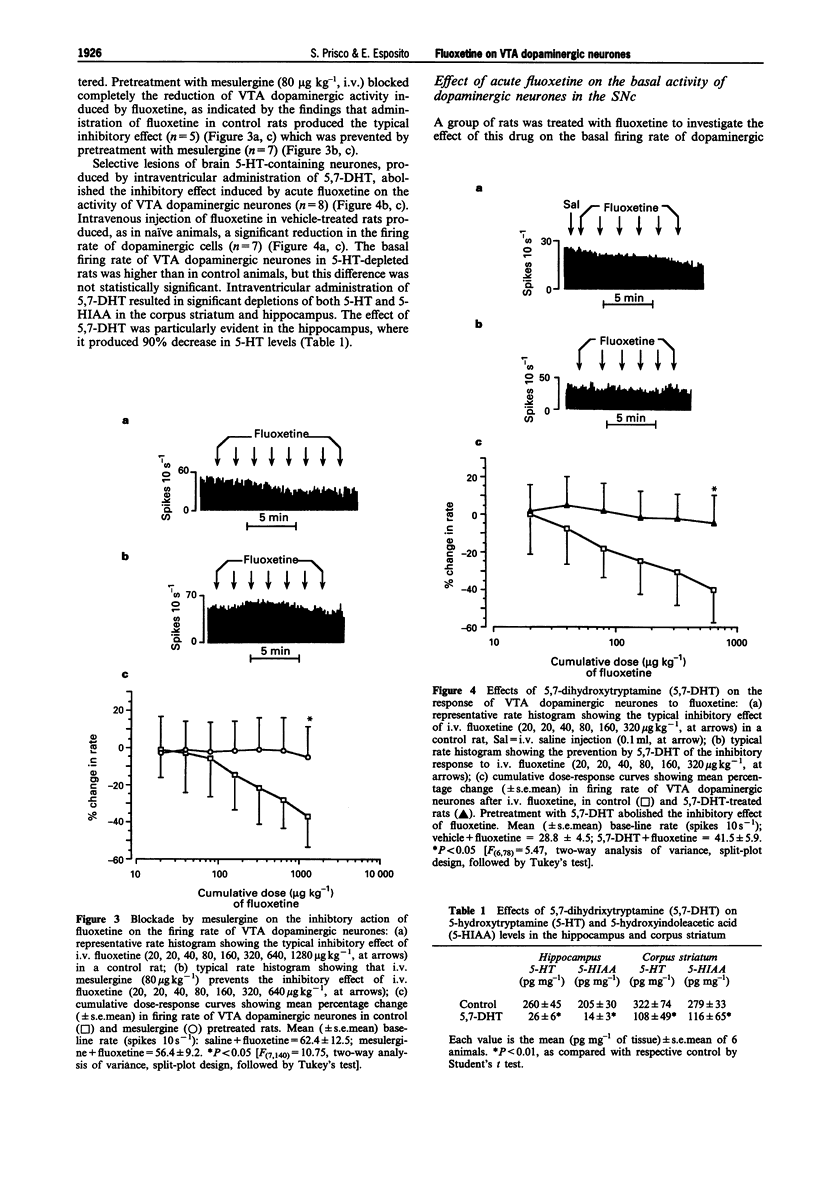
1. Electrophysiological techniques were used to study the effects of fluoxetine and citalopram on the basal activity of dopaminergic neurones in the ventral tegmental area (VTA) and substantia nigra, pars compacta (SNc) of rats. 2. Acute i.v. injection of fluoxetine (20-1280 micrograms kg-1) caused a dose-dependent inhibition of the firing rate of VTA dopaminergic neurones, but did not affect the activity of dopaminergic cells in the SNc. Citalopram (20-1280 micrograms kg-1, i.v.) inhibited the firing rate of dopaminergic neurones in the VTA, but its effect (maximal inhibition: 14 +/- 7%) was less pronounced than that of fluoxetine (maximal inhibition: 34 +/- 7%). 3. Pretreatment with mesulergine (80 micrograms kg-1, i.v.), a 5-hydroxytryptamine2C/2B (5-HT2C/2B) receptor antagonist, blocked the inhibitory effect of fluoxetine on VTA dopaminergic cells. Selective lesions of 5-hydroxytryptaminergic neurones by the neurotoxin, 5,7-dihydroxytryptamine (5,7-DHT), abolished the fluoxetine-induced reduction of VTA dopaminergic activity. 4. In a series of experiments, fluoxetine (10 mg kg-1, i.p.) was administered once daily for 21 consecutive days. Acute i.v. administration of fluoxetine (20-1280 micrograms kg-1, 72 h after the last i.p. injection) did not cause any change in the basal firing rate of VTA dopaminergic neurones in treated rats, whereas it induced the typical inhibitory effect in control animals. A group of rats chronically treated with fluoxetine, received i.v. m-chlorophenylpiperazine (mCPP; 10-320 micrograms kg-1), a 5-HT2C/2B receptor agonist. This drug significantly inhibited VTA dopaminergic function in control rats, but did not modify the basal activity of dopaminergic cells in animals given chronic fluoxetine.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aghajanian G. K. LSD and 2-bromo-LSD: comparison on effects on serotonergic neurones and on neurones in two serotonergic projection areas, the ventral lateral geniculate and amygdala. Neuropharmacology. 1976 Sep;15(9):521–528. doi: 10.1016/0028-3908(76)90102-7. [DOI] [PubMed] [Google Scholar]
- Azmitia E. C., Segal M. An autoradiographic analysis of the differential ascending projections of the dorsal and median raphe nuclei in the rat. J Comp Neurol. 1978 Jun 1;179(3):641–667. doi: 10.1002/cne.901790311. [DOI] [PubMed] [Google Scholar]
- Baldessarini R. J., Marsh E. R., Kula N. S. Interactions of fluoxetine with metabolism of dopamine and serotonin in rat brain regions. Brain Res. 1992 May 1;579(1):152–156. doi: 10.1016/0006-8993(92)90754-w. [DOI] [PubMed] [Google Scholar]
- Baldessarini R. J., Marsh E. Fluoxetine and side effects. Arch Gen Psychiatry. 1990 Feb;47(2):191–192. doi: 10.1001/archpsyc.1990.01810140091015. [DOI] [PubMed] [Google Scholar]
- Baldwin D., Fineberg N., Montgomery S. Fluoxetine, fluvoxamine and extrapyramidal tract disorders. Int Clin Psychopharmacol. 1991 Spring;6(1):51–58. doi: 10.1097/00004850-199100610-00007. [DOI] [PubMed] [Google Scholar]
- Baumgarten H. G., Björklund A., Lachenmayer L., Nobin A. Evaluation of the effects of 5,7-dihydroxytryptamine on serotonin and catecholamine neurons in the rat CNS. Acta Physiol Scand Suppl. 1973;391:1–19. [PubMed] [Google Scholar]
- Bunney B. S., Walters J. R., Roth R. H., Aghajanian G. K. Dopaminergic neurons: effect of antipsychotic drugs and amphetamine on single cell activity. J Pharmacol Exp Ther. 1973 Jun;185(3):560–571. [PubMed] [Google Scholar]
- Caccia S., Fracasso C., Garattini S., Guiso G., Sarati S. Effects of short- and long-term administration of fluoxetine on the monoamine content of rat brain. Neuropharmacology. 1992 Apr;31(4):343–347. doi: 10.1016/0028-3908(92)90066-x. [DOI] [PubMed] [Google Scholar]
- Cervo L., Grignaschi G., Samanin R. The role of the mesolimbic dopaminergic system in the desipramine effect in the forced swimming test. Eur J Pharmacol. 1990 Mar 13;178(1):129–133. doi: 10.1016/0014-2999(90)94805-8. [DOI] [PubMed] [Google Scholar]
- Cervo L., Samanin R. Evidence that dopamine mechanisms in the nucleus accumbens are selectively involved in the effect of desipramine in the forced swimming test. Neuropharmacology. 1987 Oct;26(10):1469–1472. doi: 10.1016/0028-3908(87)90165-1. [DOI] [PubMed] [Google Scholar]
- Cervo L., Samanin R. Repeated treatment with imipramine and amitriptyline reduced the immobility of rats in the swimming test by enhancing dopamine mechanisms in the nucleus accumbens. J Pharm Pharmacol. 1988 Feb;40(2):155–156. doi: 10.1111/j.2042-7158.1988.tb05208.x. [DOI] [PubMed] [Google Scholar]
- Cesana R., Ceci A., Ciprandi C., Borsini F. Mesulergine antagonism towards the fluoxetine anti-immobility effect in the forced swimming test in mice. J Pharm Pharmacol. 1993 May;45(5):473–475. doi: 10.1111/j.2042-7158.1993.tb05578.x. [DOI] [PubMed] [Google Scholar]
- Chaput Y., de Montigny C., Blier P. Effects of a selective 5-HT reuptake blocker, citalopram, on the sensitivity of 5-HT autoreceptors: electrophysiological studies in the rat brain. Naunyn Schmiedebergs Arch Pharmacol. 1986 Aug;333(4):342–348. doi: 10.1007/BF00500007. [DOI] [PubMed] [Google Scholar]
- Chouinard G. A double-blind controlled clinical trial of fluoxetine and amitriptyline in the treatment of outpatients with major depressive disorder. J Clin Psychiatry. 1985 Mar;46(3 Pt 2):32–37. [PubMed] [Google Scholar]
- Cunningham K. A., Lakoski J. M. The interaction of cocaine with serotonin dorsal raphe neurons. Single-unit extracellular recording studies. Neuropsychopharmacology. 1990 Feb;3(1):41–50. [PubMed] [Google Scholar]
- Curzon G., Kennett G. A. m-CPP: a tool for studying behavioural responses associated with 5-HT1c receptors. Trends Pharmacol Sci. 1990 May;11(5):181–182. doi: 10.1016/0165-6147(90)90109-l. [DOI] [PubMed] [Google Scholar]
- DeLean A., Munson P. J., Rodbard D. Simultaneous analysis of families of sigmoidal curves: application to bioassay, radioligand assay, and physiological dose-response curves. Am J Physiol. 1978 Aug;235(2):E97–102. doi: 10.1152/ajpendo.1978.235.2.E97. [DOI] [PubMed] [Google Scholar]
- Fontaine R., Chouinard G. An open clinical trial of fluoxetine in the treatment of obsessive-compulsive disorder. J Clin Psychopharmacol. 1986 Apr;6(2):98–101. [PubMed] [Google Scholar]
- Goodman W. K., McDougle C. J., Price L. H. The role of serotonin and dopamine in the pathophysiology of obsessive compulsive disorder. Int Clin Psychopharmacol. 1992 Jun;7 (Suppl 1):35–38. doi: 10.1097/00004850-199206001-00009. [DOI] [PubMed] [Google Scholar]
- Grace A. A., Bunney B. S. Nigral dopamine neurons: intracellular recording and identification with L-dopa injection and histofluorescence. Science. 1980 Nov 7;210(4470):654–656. doi: 10.1126/science.7433992. [DOI] [PubMed] [Google Scholar]
- Grace A. A., Bunney B. S. The control of firing pattern in nigral dopamine neurons: burst firing. J Neurosci. 1984 Nov;4(11):2877–2890. doi: 10.1523/JNEUROSCI.04-11-02877.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gram L. Fluoxetine. N Engl J Med. 1994 Nov 17;331(20):1354–1361. doi: 10.1056/NEJM199411173312008. [DOI] [PubMed] [Google Scholar]
- Hollander E., DeCaria C., Gully R., Nitescu A., Suckow R. F., Gorman J. M., Klein D. F., Liebowitz M. R. Effects of chronic fluoxetine treatment on behavioral and neuroendocrine responses to meta-chlorophenylpiperazine in obsessive-compulsive disorder. Psychiatry Res. 1991 Jan;36(1):1–17. doi: 10.1016/0165-1781(91)90113-4. [DOI] [PubMed] [Google Scholar]
- Hoyer D. Functional correlates of serotonin 5-HT1 recognition sites. J Recept Res. 1988;8(1-4):59–81. doi: 10.3109/10799898809048978. [DOI] [PubMed] [Google Scholar]
- Hrdina P. D. Regulation of high- and low-affinity [3H]imipramine recognition sites in rat brain by chronic treatment with antidepressants. Eur J Pharmacol. 1987 Jun 19;138(2):159–168. doi: 10.1016/0014-2999(87)90429-8. [DOI] [PubMed] [Google Scholar]
- Hyttel J. Citalopram--pharmacological profile of a specific serotonin uptake inhibitor with antidepressant activity. Prog Neuropsychopharmacol Biol Psychiatry. 1982;6(3):277–295. doi: 10.1016/s0278-5846(82)80179-6. [DOI] [PubMed] [Google Scholar]
- Invernizzi R., Belli S., Samanin R. Citalopram's ability to increase the extracellular concentrations of serotonin in the dorsal raphe prevents the drug's effect in the frontal cortex. Brain Res. 1992 Jul 3;584(1-2):322–324. doi: 10.1016/0006-8993(92)90914-u. [DOI] [PubMed] [Google Scholar]
- Jenike M. A., Baer L., Greist J. H. Clomipramine versus fluoxetine in obsessive-compulsive disorder: a retrospective comparison of side effects and efficacy. J Clin Psychopharmacol. 1990 Apr;10(2):122–124. doi: 10.1097/00004714-199004000-00008. [DOI] [PubMed] [Google Scholar]
- Kaye W. H., Weltzin T. E., Hsu L. K., Bulik C. M. An open trial of fluoxetine in patients with anorexia nervosa. J Clin Psychiatry. 1991 Nov;52(11):464–471. [PubMed] [Google Scholar]
- Kelland M. D., Freeman A. S., Chiodo L. A. Serotonergic afferent regulation of the basic physiology and pharmacological responsiveness of nigrostriatal dopamine neurons. J Pharmacol Exp Ther. 1990 May;253(2):803–811. [PubMed] [Google Scholar]
- Kennedy A. J., Gibson E. L., O'Connell M. T., Curzon G. Effects of housing, restraint and chronic treatments with mCPP and sertraline on behavioural responses to mCPP. Psychopharmacology (Berl) 1993;113(2):262–268. doi: 10.1007/BF02245708. [DOI] [PubMed] [Google Scholar]
- Kennett G. A., Curzon G. Evidence that mCPP may have behavioural effects mediated by central 5-HT1C receptors. Br J Pharmacol. 1988 May;94(1):137–147. doi: 10.1111/j.1476-5381.1988.tb11508.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennett G. A., Lightowler S., de Biasi V., Stevens N. C., Wood M. D., Tulloch I. F., Blackburn T. P. Effect of chronic administration of selective 5-hydroxytryptamine and noradrenaline uptake inhibitors on a putative index of 5-HT2C/2B receptor function. Neuropharmacology. 1994 Dec;33(12):1581–1588. doi: 10.1016/0028-3908(94)90133-3. [DOI] [PubMed] [Google Scholar]
- Kennett G. A., Wood M. D., Glen A., Grewal S., Forbes I., Gadre A., Blackburn T. P. In vivo properties of SB 200646A, a 5-HT2C/2B receptor antagonist. Br J Pharmacol. 1994 Mar;111(3):797–802. doi: 10.1111/j.1476-5381.1994.tb14808.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine R., Hoffman J. S., Knepple E. D., Kenin M. Long-term fluoxetine treatment of a large number of obsessive-compulsive patients. J Clin Psychopharmacol. 1989 Aug;9(4):281–283. [PubMed] [Google Scholar]
- Lipinski J. F., Jr, Mallya G., Zimmerman P., Pope H. G., Jr Fluoxetine-induced akathisia: clinical and theoretical implications. J Clin Psychiatry. 1989 Sep;50(9):339–342. [PubMed] [Google Scholar]
- Maj J., Moryl E. Effects of sertraline and citalopram given repeatedly on the responsiveness of 5-HT receptor subpopulations. J Neural Transm Gen Sect. 1992;88(2):143–156. doi: 10.1007/BF01244819. [DOI] [PubMed] [Google Scholar]
- Marsden C. D., Jenner P. The pathophysiology of extrapyramidal side-effects of neuroleptic drugs. Psychol Med. 1980 Feb;10(1):55–72. doi: 10.1017/s003329170003960x. [DOI] [PubMed] [Google Scholar]
- Meltzer H. Y., Young M., Metz J., Fang V. S., Schyve P. M., Arora R. C. Extrapyramidal side effects and increased serum prolactin following fluoxetine, a new antidepressant. J Neural Transm. 1979;45(2):165–175. doi: 10.1007/BF01250091. [DOI] [PubMed] [Google Scholar]
- Moore R. Y., Bloom F. E. Central catecholamine neuron systems: anatomy and physiology of the dopamine systems. Annu Rev Neurosci. 1978;1:129–169. doi: 10.1146/annurev.ne.01.030178.001021. [DOI] [PubMed] [Google Scholar]
- Mori S., Matsuura T., Takino T., Sano Y. Light and electron microscopic immunohistochemical studies of serotonin nerve fibers in the substantia nigra of the rat, cat and monkey. Anat Embryol (Berl) 1987;176(1):13–18. doi: 10.1007/BF00309747. [DOI] [PubMed] [Google Scholar]
- Perry K. W., Fuller R. W. Effect of fluoxetine on serotonin and dopamine concentration in microdialysis fluid from rat striatum. Life Sci. 1992;50(22):1683–1690. doi: 10.1016/0024-3205(92)90423-m. [DOI] [PubMed] [Google Scholar]
- Perry K. W., Fuller R. W. Extracellular 5-hydroxytryptamine concentration in rat hypothalamus after administration of fluoxetine plus L-5-hydroxytryptophan. J Pharm Pharmacol. 1993 Aug;45(8):759–761. doi: 10.1111/j.2042-7158.1993.tb07105.x. [DOI] [PubMed] [Google Scholar]
- Phillipson O. T. Afferent projections to the ventral tegmental area of Tsai and interfascicular nucleus: a horseradish peroxidase study in the rat. J Comp Neurol. 1979 Sep 1;187(1):117–143. doi: 10.1002/cne.901870108. [DOI] [PubMed] [Google Scholar]
- Prisco S., Cagnotto A., Talone D., De Blasi A., Mennini T., Esposito E. Tertatolol, a new beta-blocker, is a serotonin (5-hydroxytryptamine1A) receptor antagonist in rat brain. J Pharmacol Exp Ther. 1993 May;265(2):739–744. [PubMed] [Google Scholar]
- Prisco S., Pagannone S., Esposito E. Serotonin-dopamine interaction in the rat ventral tegmental area: an electrophysiological study in vivo. J Pharmacol Exp Ther. 1994 Oct;271(1):83–90. [PubMed] [Google Scholar]
- Rutter J. J., Auerbach S. B. Acute uptake inhibition increases extracellular serotonin in the rat forebrain. J Pharmacol Exp Ther. 1993 Jun;265(3):1319–1324. [PubMed] [Google Scholar]
- Samanin R., Bernasconi S., Garattini S. The effect of nomifensine on the depletion of brain serotonin and catecholamines induced respectively by fenfluramine and 6-hydroxydopamine in rats. Eur J Pharmacol. 1975 Dec;34(2):377–380. doi: 10.1016/0014-2999(75)90266-6. [DOI] [PubMed] [Google Scholar]
- Sinton C. M., Fallon S. L. Electrophysiological evidence for a functional differentiation between subtypes of the 5-HT1 receptor. Eur J Pharmacol. 1988 Nov 22;157(2-3):173–181. doi: 10.1016/0014-2999(88)90380-9. [DOI] [PubMed] [Google Scholar]
- Stark P., Fuller R. W., Wong D. T. The pharmacologic profile of fluoxetine. J Clin Psychiatry. 1985 Mar;46(3 Pt 2):7–13. [PubMed] [Google Scholar]
- Stark P., Hardison C. D. A review of multicenter controlled studies of fluoxetine vs. imipramine and placebo in outpatients with major depressive disorder. J Clin Psychiatry. 1985 Mar;46(3 Pt 2):53–58. [PubMed] [Google Scholar]
- Tanda G., Carboni E., Frau R., Di Chiara G. Increase of extracellular dopamine in the prefrontal cortex: a trait of drugs with antidepressant potential? Psychopharmacology (Berl) 1994 Jun;115(1-2):285–288. doi: 10.1007/BF02244785. [DOI] [PubMed] [Google Scholar]
- Tate J. L. Extrapyramidal symptoms in a patient taking haloperidol and fluoxetine. Am J Psychiatry. 1989 Mar;146(3):399–400. doi: 10.1176/ajp.146.3.399b. [DOI] [PubMed] [Google Scholar]
- Thomas D. R., Nelson D. R., Johnson A. M. Biochemical effects of the antidepressant paroxetine, a specific 5-hydroxytryptamine uptake inhibitor. Psychopharmacology (Berl) 1987;93(2):193–200. doi: 10.1007/BF00179933. [DOI] [PubMed] [Google Scholar]
- Turner S. M., Jacob R. G., Beidel D. C., Himmelhoch J. Fluoxetine treatment of obsessive-compulsive disorder. J Clin Psychopharmacol. 1985 Aug;5(4):207–212. [PubMed] [Google Scholar]
- White R. S. Psychotherapy with schizophrenic patients. Am J Psychiatry. 1989 Oct;146(10):1353–1354. doi: 10.1176/ajp.146.10.aj146101353. [DOI] [PubMed] [Google Scholar]
- Wong D. T., Horng J. S., Bymaster F. P., Hauser K. L., Molloy B. B. A selective inhibitor of serotonin uptake: Lilly 110140, 3-(p-trifluoromethylphenoxy)-N-methyl-3-phenylpropylamine. Life Sci. 1974 Aug 1;15(3):471–479. doi: 10.1016/0024-3205(74)90345-2. [DOI] [PubMed] [Google Scholar]
- Wong D. T., Threlkeld P. G., Robertson D. W. Affinities of fluoxetine, its enantiomers, and other inhibitors of serotonin uptake for subtypes of serotonin receptors. Neuropsychopharmacology. 1991 Aug;5(1):43–47. [PubMed] [Google Scholar]



