Abstract
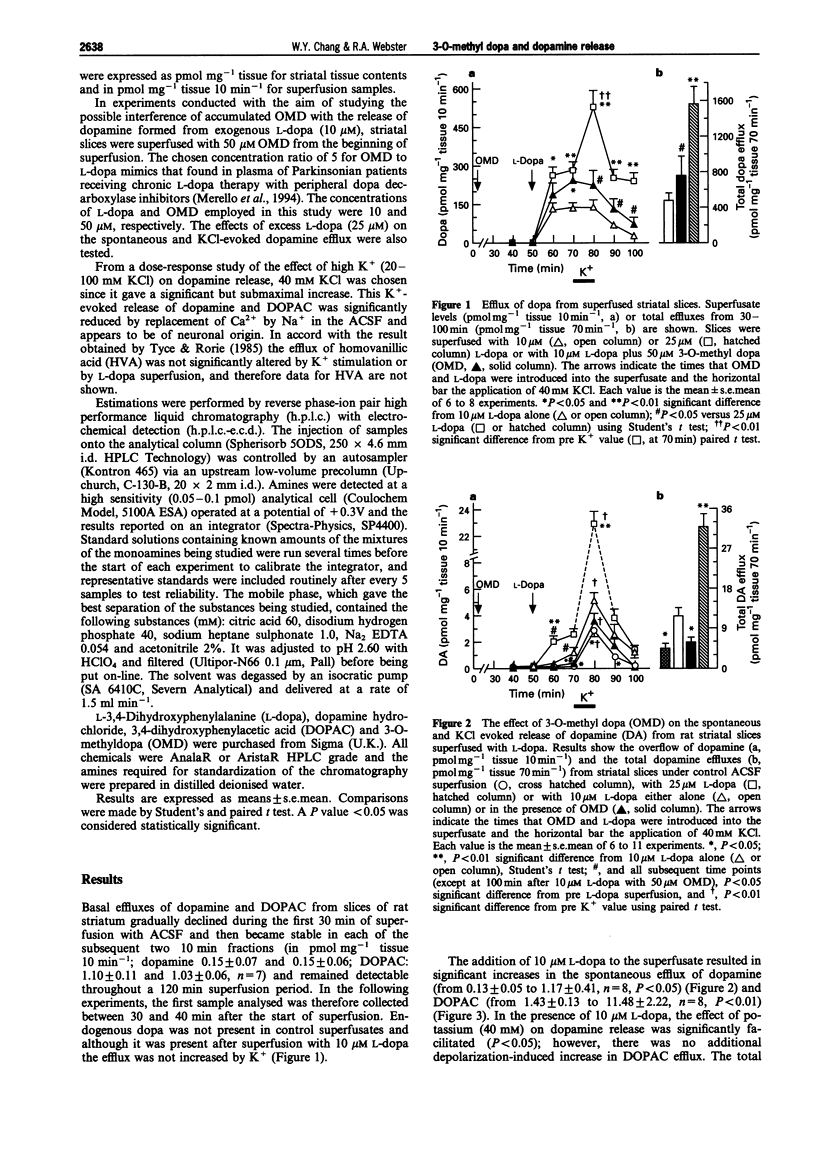
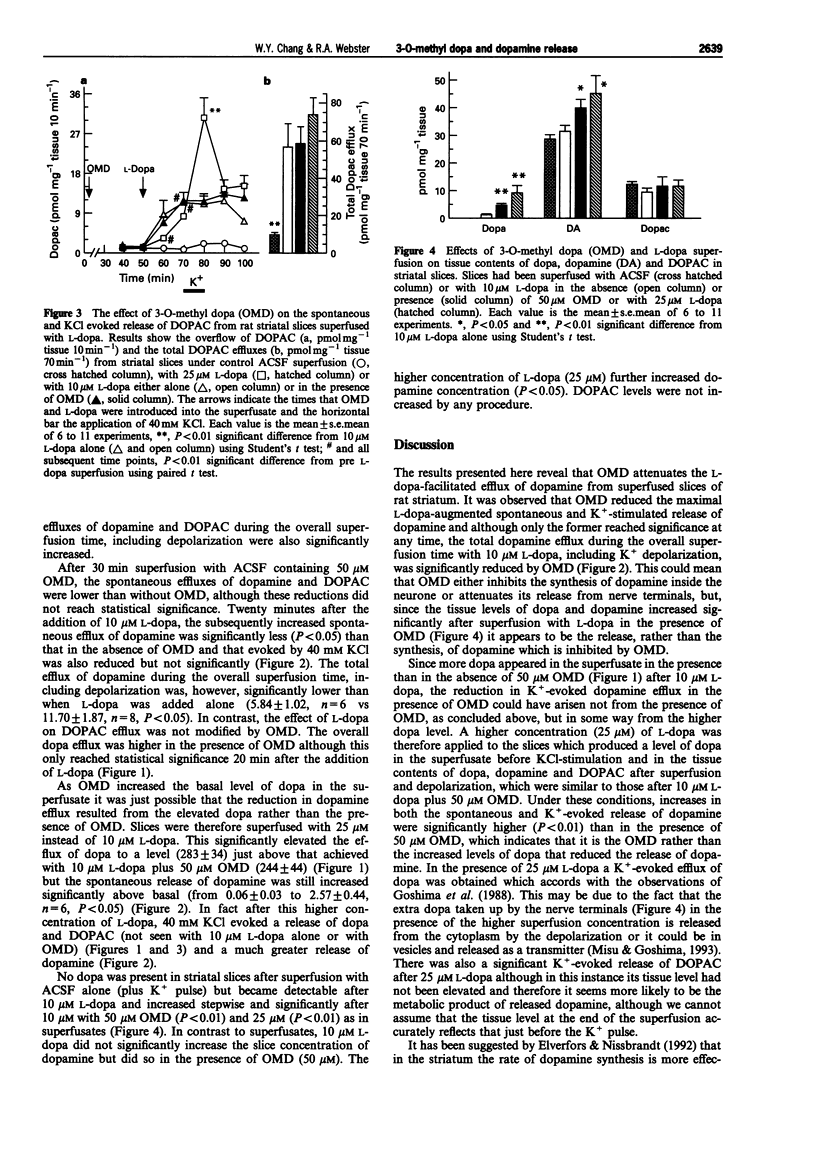
1. The effect of L-dopa on the spontaneous and KCl-evoked efflux of dopamine from rat striatal slices, measured by high performance liquid chromatography (h.p.l.c.) with electrochemical detection (e.c.d.) was investigated in the absence and presence of 3-O-methyl dopa (OMD), an O-methylated metabolite of L-dopa. 2. The addition of exogenous L-dopa (10 microM) significantly increased both the spontaneous efflux of dopamine and that evoked by KCl. 3. In the presence of 50 microM OMD, the effects of L-dopa on the spontaneous and KCl-evoked efflux of dopamine were smaller but only the former was significantly different from that in the absence of OMD. However, the total efflux of dopamine during the overall superfusion time (70 min) including KCl depolarization was significantly lower than in the absence of OMD. 4. Analysis of tissue content after superfusion revealed that the levels of dopa and dopamine in slices superfused with L-dopa in the presence of OMD were significantly higher than those superfused with L-dopa alone. 5. The finding that OMD significantly reduced the efflux of dopamine whilst increasing its concentration in striatal slices after L-dopa superfusion could explain the reduced efficacy seen after long-term therapy with L-dopa and a peripheral dopa decarboxylase inhibitor in Parkinsonian patients when plasma and brain OMD are very high.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartholini G., Kuruma I., Pletscher A. The metabolic pathways of L-3-O-methyldopa. J Pharmacol Exp Ther. 1972 Oct;183(1):65–72. [PubMed] [Google Scholar]
- Becker J. B., Castañeda E., Robinson T. E., Beer M. E. A simple in vitro technique to measure the release of endogenous dopamine and dihydroxyphenylacetic acid from striatal tissue using high performance liquid chromatography with electrochemical detection. J Neurosci Methods. 1984 May;11(1):19–28. doi: 10.1016/0165-0270(84)90004-9. [DOI] [PubMed] [Google Scholar]
- Elverfors A., Nissbrandt H. Effects of d-amphetamine on dopaminergic neurotransmission; a comparison between the substantia nigra and the striatum. Neuropharmacology. 1992 Jul;31(7):661–670. doi: 10.1016/0028-3908(92)90144-e. [DOI] [PubMed] [Google Scholar]
- Fabbrini G., Juncos J., Mouradian M. M., Serrati C., Chase T. N. Levodopa pharmacokinetic mechanisms and motor fluctuations in Parkinson's disease. Ann Neurol. 1987 Apr;21(4):370–376. doi: 10.1002/ana.410210409. [DOI] [PubMed] [Google Scholar]
- Gervas J. J., Muradás V., Bazán E., Aguado E. G., de Yébenes J. G. Effects of 3-OM-dopa on monoamine metabolism in rat brain. Neurology. 1983 Mar;33(3):278–282. doi: 10.1212/wnl.33.3.278. [DOI] [PubMed] [Google Scholar]
- Goshima Y., Kubo T., Misu Y. Transmitter-like release of endogenous 3,4-dihydroxyphenylalanine from rat striatal slices. J Neurochem. 1988 Jun;50(6):1725–1730. doi: 10.1111/j.1471-4159.1988.tb02470.x. [DOI] [PubMed] [Google Scholar]
- Hardie R. J. Problems and unanswered questions concerning levodopa treatment in Parkinson's disease. Acta Neurol Scand Suppl. 1989;126:77–82. doi: 10.1111/j.1600-0404.1989.tb01786.x. [DOI] [PubMed] [Google Scholar]
- Kuruma I., Bartholini G., Tissot R., Pletscher A. The metabolism of L-3-O-methyldopa, a precursor of dopa in man. Clin Pharmacol Ther. 1971 Jul-Aug;12(4):678–682. doi: 10.1002/cpt1971124678. [DOI] [PubMed] [Google Scholar]
- Merello M., Lees A. J., Webster R., Bovingdon M., Gordin A. Effect of entacapone, a peripherally acting catechol-O-methyltransferase inhibitor, on the motor response to acute treatment with levodopa in patients with Parkinson's disease. J Neurol Neurosurg Psychiatry. 1994 Feb;57(2):186–189. doi: 10.1136/jnnp.57.2.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misu Y., Goshima Y. Is L-dopa an endogenous neurotransmitter? Trends Pharmacol Sci. 1993 Apr;14(4):119–123. doi: 10.1016/0165-6147(93)90082-u. [DOI] [PubMed] [Google Scholar]
- Patel J., Trout S. J., Kruk Z. L. Regional differences in evoked dopamine efflux in brain slices of rat anterior and posterior caudate putamen. Naunyn Schmiedebergs Arch Pharmacol. 1992 Sep;346(3):267–276. doi: 10.1007/BF00173539. [DOI] [PubMed] [Google Scholar]
- Snyder G. L., Zigmond M. J. The effects of L-dopa on in vitro dopamine release from striatum. Brain Res. 1990 Feb 5;508(2):181–187. doi: 10.1016/0006-8993(90)90394-q. [DOI] [PubMed] [Google Scholar]
- Starke K., Göthert M., Kilbinger H. Modulation of neurotransmitter release by presynaptic autoreceptors. Physiol Rev. 1989 Jul;69(3):864–989. doi: 10.1152/physrev.1989.69.3.864. [DOI] [PubMed] [Google Scholar]
- Suaud-Chagny M. F., Ponec J., Gonon F. Presynaptic autoinhibition of the electrically evoked dopamine release studied in the rat olfactory tubercle by in vivo electrochemistry. Neuroscience. 1991;45(3):641–652. doi: 10.1016/0306-4522(91)90277-u. [DOI] [PubMed] [Google Scholar]
- Tohgi H., Abe T., Kikuchi T., Takahashi S., Nozaki Y. The significance of 3-O-methyldopa concentrations in the cerebrospinal fluid in the pathogenesis of wearing-off phenomenon in Parkinson's disease. Neurosci Lett. 1991 Oct 28;132(1):19–22. doi: 10.1016/0304-3940(91)90422-p. [DOI] [PubMed] [Google Scholar]
- Tyce G. M., Rorie D. K. Effects of L-dopa and L-tyrosine on release of free and conjugated dopamine, homovanillic acid and dihydroxyphenylacetic acid from slices of rat striatum. Life Sci. 1985 Dec 23;37(25):2439–2448. doi: 10.1016/0024-3205(85)90112-2. [DOI] [PubMed] [Google Scholar]



