Abstract
1. In the present study we assessed the formation of nitric oxide (NO) from classical and thiol-containing organic nitrates in vascular tissues and organs of anaesthetized rabbits, and established a relationship between the relaxant response elicited by nitroglycerin (NTG) and NO formation in the rabbit isolated aorta. Furthermore, the effect of isolated cytochrome P450 on NO formation from organic nitrates was investigated. 2. Rabbits received diethyldithiocarbamate (DETC; 200 mg kg-1 initial bolus i.p. and 200 mg kg-1 during 20 min, i.v.) and either saline, or one of the following organic nitrates: nitroglycerin (NTG, 0.5 mg kg-1), isosorbide dinitrate (ISDN), N-(3-nitratopivaloyl)-L-cysteine ethylester (SPM 3672), S-carboxyethyl-N-(3-nitratopivaloyl)-L-cysteine ethylester (SPM 5185), at 10 mg kg-1 each. After 20 min the animals were killed, blood vessels and organs were removed, and subsequently analyzed for spin-trapped NO by cryogenic electron spin resonance (e.s.r.) spectroscopy. 3. In the saline-treated control group, NO remained below the detection limit in all vessels and organs. In contrast, all of the nitrates tested elicited measurable NO formation, which was higher in organs (liver, kidney, heart, lung, spleen) (up to 4.8 nmol g-1 20 min-1) than in blood vessels (vena cava, mesenteric bed, femoral artery, aorta) (up to 0.7 nmol g-1 20 min-1). Classical organic nitrates (NTG, ISDN) formed NO preferentially in the mesenteric bed and the vena cava, while the SPM compounds elicited comparable NO formation in veins and arteries. 4. Using a similar spin trapping technique, NO formation was assessed in vitro in phenylephrine-precontracted rabbit aortic rings. The maximal relaxation elicited by a first exposure (10 min) to NTG (0.3 to 10 microM) was positively correlated (r = 0.8) with the net increase (NTG minus basal) of NO spin-trapped during a second exposure to the same concentration of NTG in the presence of DETC. 5. Cytochrome P450 purified from rabbit liver enhanced NO formation in a NADPH-dependent fashion from NTG, but not from the other nitrates, as assessed by activation of purified soluble guanylyl cyclase. 6. We conclude that the vessel selective action of different organic nitrates in vivo reflects differences in vascular NO formation. Thus, efficient preload reduction by classical organic nitrates can be accounted for by higher NO formation in venous capacitance as compared to arterial conductance and resistance vessels. In contrast, NO is released from cysteine-containing nitrates (SPMs) to a similar extent in arteries and veins, presumably independently of an organic nitrate-specific biotransformation. Limited tissue bioavailability of NTG and ISDN might account for low NO formation in the aorta, while true differences in biotransformation seem to account for differences in NO formation in the other vascular tissues.
Full text
PDF

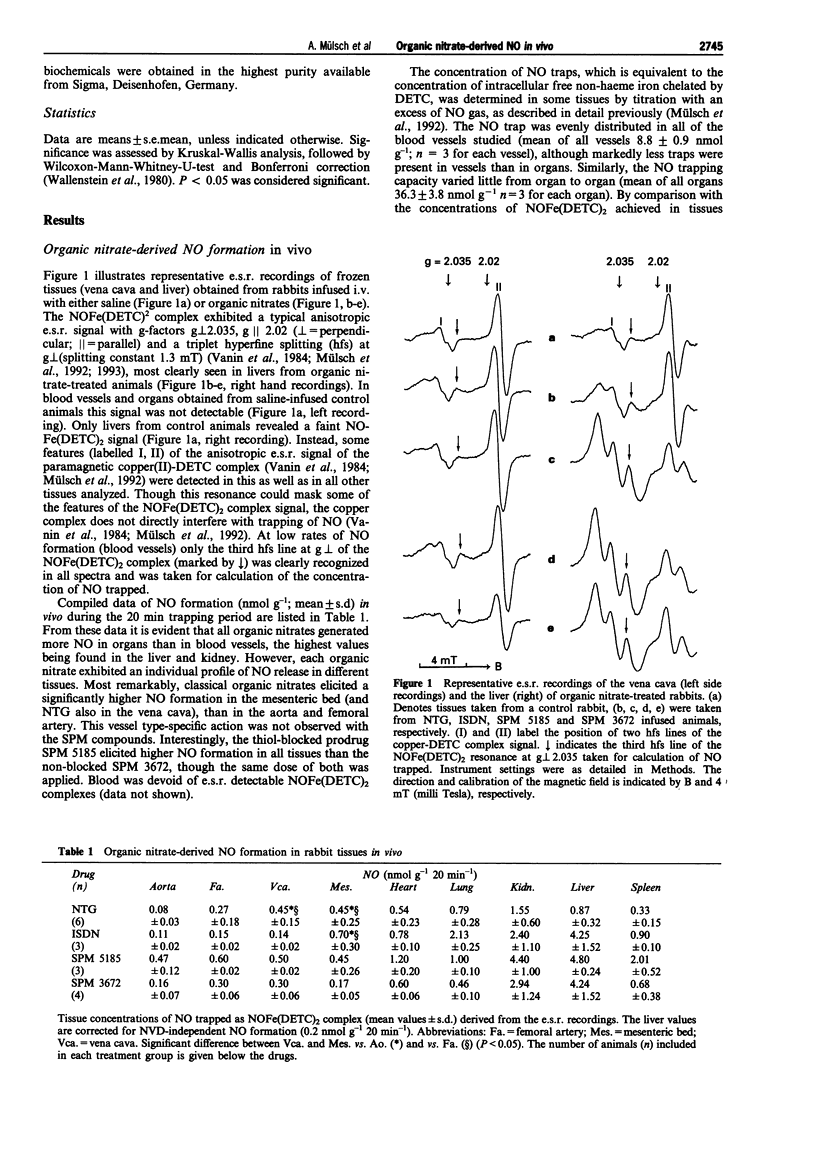
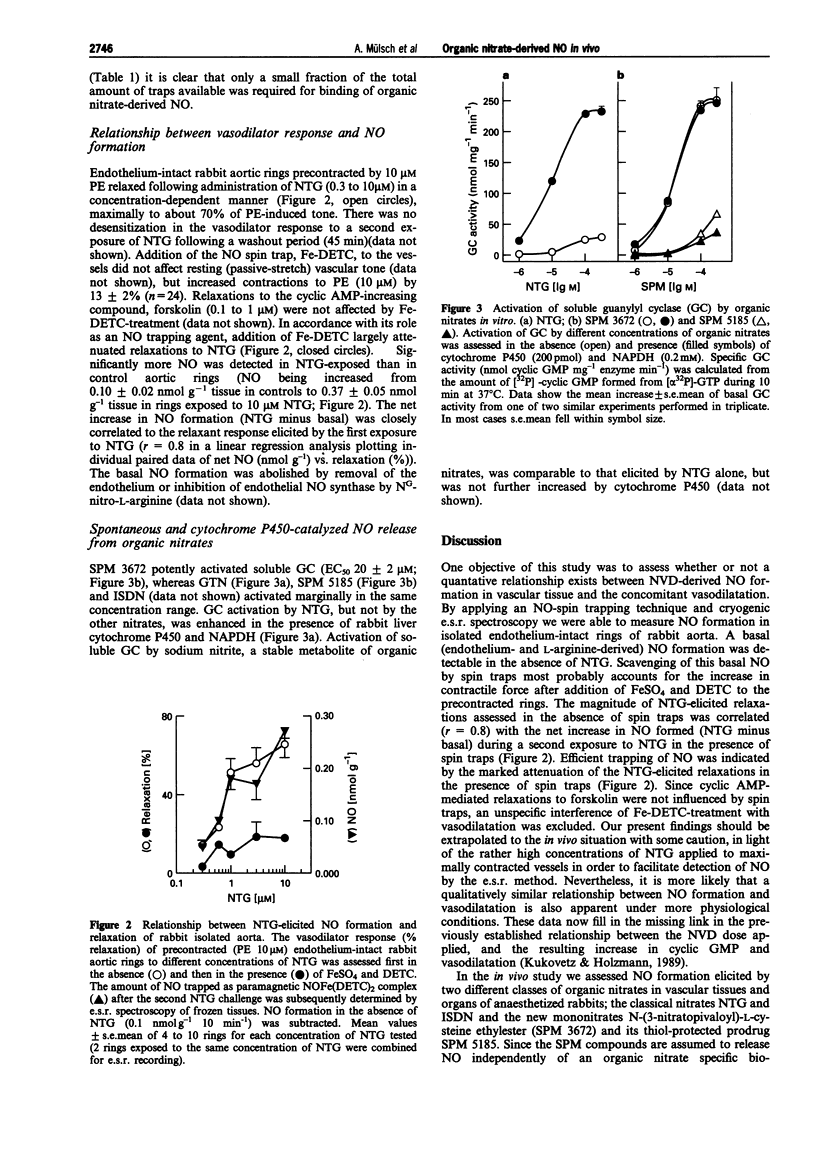



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahlner J., Andersson R. G., Torfgård K., Axelsson K. L. Organic nitrate esters: clinical use and mechanisms of actions. Pharmacol Rev. 1991 Sep;43(3):351–423. [PubMed] [Google Scholar]
- Armstrong P. W., Moffat J. A., Marks G. S. Arterial-venous nitroglycerin gradient during intravenous infusion in man. Circulation. 1982 Dec;66(6):1273–1276. doi: 10.1161/01.cir.66.6.1273. [DOI] [PubMed] [Google Scholar]
- Bennett B. M., McDonald B. J., Nigam R., Long P. G., Simon W. C. Inhibition of nitrovasodilator- and acetylcholine-induced relaxation and cyclic GMP accumulation by the cytochrome P-450 substrate, 7-ethoxyresorufin. Can J Physiol Pharmacol. 1992 Sep;70(9):1297–1303. doi: 10.1139/y92-181. [DOI] [PubMed] [Google Scholar]
- Bennett B. M., McDonald B. J., Nigam R., Simon W. C. Biotransformation of organic nitrates and vascular smooth muscle cell function. Trends Pharmacol Sci. 1994 Jul;15(7):245–249. doi: 10.1016/0165-6147(94)90319-0. [DOI] [PubMed] [Google Scholar]
- Brien J. F., McLaughlin B. E., Breedon T. H., Bennett B. M., Nakatsu K., Marks G. S. Biotransformation of glyceryl trinitrate occurs concurrently with relaxation of rabbit aorta. J Pharmacol Exp Ther. 1986 May;237(2):608–614. [PubMed] [Google Scholar]
- Cleeter M. W., Cooper J. M., Darley-Usmar V. M., Moncada S., Schapira A. H. Reversible inhibition of cytochrome c oxidase, the terminal enzyme of the mitochondrial respiratory chain, by nitric oxide. Implications for neurodegenerative diseases. FEBS Lett. 1994 May 23;345(1):50–54. doi: 10.1016/0014-5793(94)00424-2. [DOI] [PubMed] [Google Scholar]
- Clement B., Jung F., Pfunder H. N-hydroxylation of benzamidine to benzamidoxime by a reconstituted cytochrome P-450 oxidase system from rabbit liver: involvement of cytochrome P-450 IIC3. Mol Pharmacol. 1993 Mar;43(3):335–342. [PubMed] [Google Scholar]
- Cossum P. A., Roberts M. S., Yong A. C., Kilpatrick D. Distribution and metabolism of nitroglycerin and its metabolites in vascular beds of sheep. J Pharmacol Exp Ther. 1986 Jun;237(3):959–966. [PubMed] [Google Scholar]
- Feelisch M., Kelm M. Biotransformation of organic nitrates to nitric oxide by vascular smooth muscle and endothelial cells. Biochem Biophys Res Commun. 1991 Oct 15;180(1):286–293. doi: 10.1016/s0006-291x(05)81290-2. [DOI] [PubMed] [Google Scholar]
- Ferrer M. I., Bradley S. E., Wheeler H. O., Enson Y., Preising R., Brickner P. W., Conroy R. J., Harvey R. M. Some effects of nitroglycerin upon the splanchinic, pulmonary, and systemic circulations. Circulation. 1966 Mar;33(3):357–373. doi: 10.1161/01.cir.33.3.357. [DOI] [PubMed] [Google Scholar]
- Fung H. L., Sutton S. C., Kamiya A. Blood vessel uptake and metabolism of organic nitrates in the rat. J Pharmacol Exp Ther. 1984 Feb;228(2):334–341. [PubMed] [Google Scholar]
- Imhof P. R., Ott B., Frankhauser P., Chu L. C., Hodler J. Difference in nitroglycerin dose-response in the venous and arterial beds. Eur J Clin Pharmacol. 1980 Nov;18(6):455–460. doi: 10.1007/BF00874655. [DOI] [PubMed] [Google Scholar]
- Kojda G., Noack E. Nitric oxide liberating, soluble guanylate cyclase stimulating and vasorelaxing properties of the new nitrate-compound SPM 3672. J Cardiovasc Pharmacol. 1993 Jul;22(1):103–111. doi: 10.1097/00005344-199307000-00017. [DOI] [PubMed] [Google Scholar]
- Kukovetz W. R., Holzmann S. Tolerance and cross-tolerance between SIN-1 and nitric oxide in bovine coronary arteries. J Cardiovasc Pharmacol. 1989;14 (Suppl 11):S40–S46. [PubMed] [Google Scholar]
- Kuropteva Z. V., Pastushenko O. N. Izmeneniia v paramagnitnykh kompleksakh krovi i pecheni zhivotnykh pod deistviem nitroglitserina. Dokl Akad Nauk SSSR. 1985 Mar-Apr;281(1):189–192. [PubMed] [Google Scholar]
- Kurz M. A., Lamping K. G., Bates J. N., Eastham C. L., Marcus M. L., Harrison D. G. Mechanisms responsible for the heterogeneous coronary microvascular response to nitroglycerin. Circ Res. 1991 Mar;68(3):847–855. doi: 10.1161/01.res.68.3.847. [DOI] [PubMed] [Google Scholar]
- Liu Z., Brien J. F., Marks G. S., McLaughlin B. E., Nakatsu K. Lack of evidence for the involvement of cytochrome P-450 or other hemoproteins in metabolic activation of glyceryl trinitrate in rabbit aorta. J Pharmacol Exp Ther. 1993 Mar;264(3):1432–1439. [PubMed] [Google Scholar]
- Loos D., Schneider R., Schörner W. Changes in regional body blood volume caused by nitroglycerin. Z Kardiol. 1983;72 (Suppl 3):29–32. [PubMed] [Google Scholar]
- Mackenzie J. E., Parratt J. R. Comparative effects of glyceryl trinitrate on venous and arterial smooth muscle in vitro; relevance to antianginal activity. Br J Pharmacol. 1977 May;60(1):155–160. doi: 10.1111/j.1476-5381.1977.tb16760.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald B. J., Bennett B. M. Biotransformation of glyceryl trinitrate by rat aortic cytochrome P450. Biochem Pharmacol. 1993 Jan 7;45(1):268–270. doi: 10.1016/0006-2952(93)90403-j. [DOI] [PubMed] [Google Scholar]
- Moncada S., Palmer R. M., Higgs E. A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991 Jun;43(2):109–142. [PubMed] [Google Scholar]
- Mülsch A., Busse R., Mordvintcev P. I., Vanin A. F., Nielsen E. O., Scheel-Krüger J., Olesen S. P. Nitric oxide promotes seizure activity in kainate-treated rats. Neuroreport. 1994 Nov 21;5(17):2325–2328. doi: 10.1097/00001756-199411000-00029. [DOI] [PubMed] [Google Scholar]
- Mülsch A., Busse R., Winter I., Bassenge E. Endothelium- and sydnonimine-induced responses of native and cultured aortic smooth muscle cells are not impaired by nitroglycerin tolerance. Naunyn Schmiedebergs Arch Pharmacol. 1989 May;339(5):568–574. doi: 10.1007/BF00167263. [DOI] [PubMed] [Google Scholar]
- Mülsch A., Gerzer R. Purification of heme-containing soluble guanylyl cyclase. Methods Enzymol. 1991;195:377–383. doi: 10.1016/0076-6879(91)95183-k. [DOI] [PubMed] [Google Scholar]
- Mülsch A., Hecker M., Mordvintcev P. I., Vanin A. F., Busse R. Enzymic and nonenzymic release of NO accounts for the vasodilator activity of the metabolites of CAS 936, a novel long-acting sydnonimine derivative. Naunyn Schmiedebergs Arch Pharmacol. 1993 Jan;347(1):92–100. doi: 10.1007/BF00168778. [DOI] [PubMed] [Google Scholar]
- Schröder H. Cytochrome P-450 mediates bioactivation of organic nitrates. J Pharmacol Exp Ther. 1992 Jul;262(1):298–302. [PubMed] [Google Scholar]
- Schröder H., Schrör K. Inhibitors of cytochrome P-450 reduce cyclic GMP stimulation by glyceryl trinitrate in LLC-PK1 kidney epithelial cells. Naunyn Schmiedebergs Arch Pharmacol. 1990 Nov;342(5):616–618. doi: 10.1007/BF00169054. [DOI] [PubMed] [Google Scholar]
- Schrör K., Förster S., Woditsch I. On-line measurement of nitric oxide release from organic nitrates in the intact coronary circulation. Naunyn Schmiedebergs Arch Pharmacol. 1991 Aug;344(2):240–246. doi: 10.1007/BF00167225. [DOI] [PubMed] [Google Scholar]
- Servent D., Delaforge M., Ducrocq C., Mansuy D., Lenfant M. Nitric oxide formation during microsomal hepatic denitration of glyceryl trinitrate: involvement of cytochrome P-450. Biochem Biophys Res Commun. 1989 Sep 29;163(3):1210–1216. doi: 10.1016/0006-291x(89)91106-6. [DOI] [PubMed] [Google Scholar]
- Seth P., Fung H. L. Biochemical characterization of a membrane-bound enzyme responsible for generating nitric oxide from nitroglycerin in vascular smooth muscle cells. Biochem Pharmacol. 1993 Oct 19;46(8):1481–1486. doi: 10.1016/0006-2952(93)90115-d. [DOI] [PubMed] [Google Scholar]
- Shepherd A. N., Hayes P. C., Jacyna M., Morrison L., Bouchier I. A. The influence of captopril, the nitrates and propranolol on apparent liver blood flow. Br J Clin Pharmacol. 1985 Mar;19(3):393–397. doi: 10.1111/j.1365-2125.1985.tb02659.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadler J., Trockfeld J., Schmalix W. A., Brill T., Siewert J. R., Greim H., Doehmer J. Inhibition of cytochromes P4501A by nitric oxide. Proc Natl Acad Sci U S A. 1994 Apr 26;91(9):3559–3563. doi: 10.1073/pnas.91.9.3559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tominaga T., Sato S., Ohnishi T., Ohnishi S. T. Electron paramagnetic resonance (EPR) detection of nitric oxide produced during forebrain ischemia of the rat. J Cereb Blood Flow Metab. 1994 Sep;14(5):715–722. doi: 10.1038/jcbfm.1994.92. [DOI] [PubMed] [Google Scholar]
- Wallenstein S., Zucker C. L., Fleiss J. L. Some statistical methods useful in circulation research. Circ Res. 1980 Jul;47(1):1–9. doi: 10.1161/01.res.47.1.1. [DOI] [PubMed] [Google Scholar]
- Zanzinger J., Feelisch M., Bassenge E. Novel organic nitrates are potent dilators of large coronary arteries with reduced development of tolerance during long-term infusion in dogs: role of the sulfhydryl moiety. J Cardiovasc Pharmacol. 1994 May;23(5):772–778. doi: 10.1097/00005344-199405000-00012. [DOI] [PubMed] [Google Scholar]


