Abstract
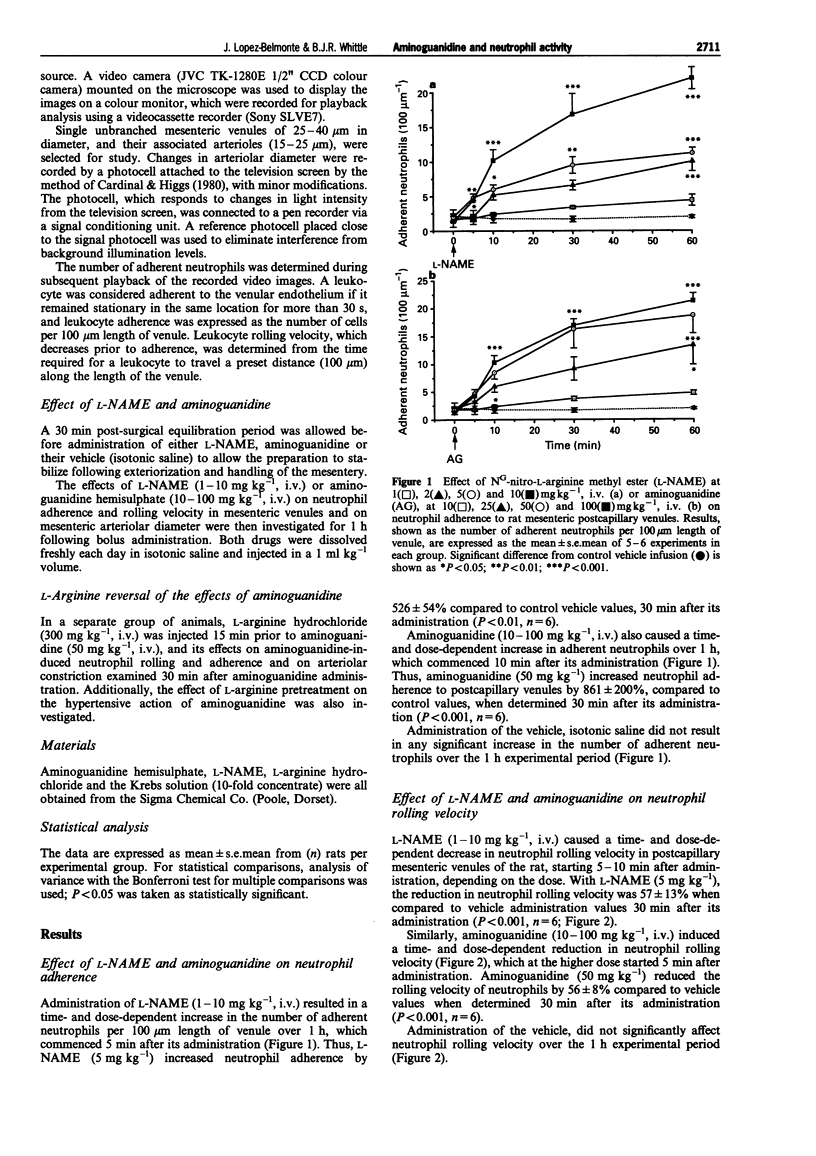
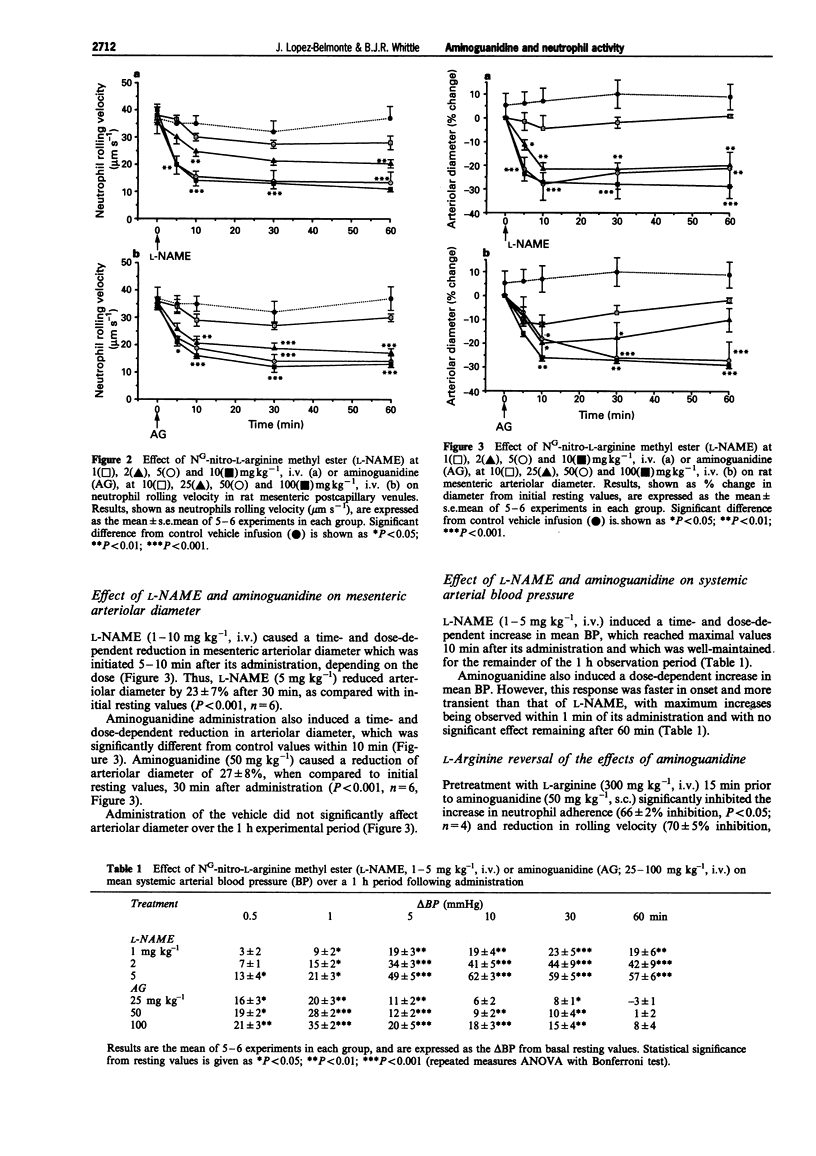
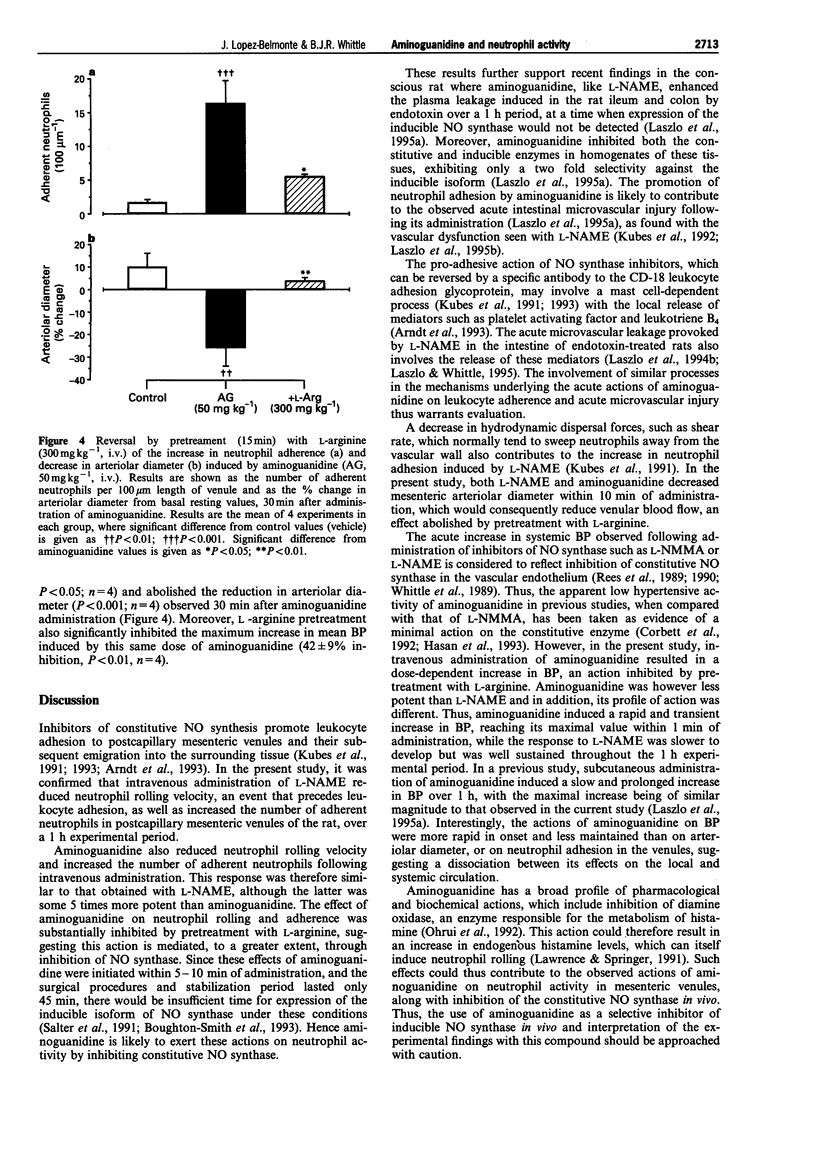
1. The effects of aminoguanidine on neutrophil adherence to venules and on the diameter of arterioles in the mesenteric vascular bed of the pentobarbitone-anaesthetized rat have been compared with those of the nitric oxide synthase inhibitor, NG-nitro-L-arginine methyl ester (L-NAME). 2. Administration of L-NAME (1-10 mg kg-1, i.v.) caused a dose-dependent increase in leukocyte adherence and a reduction in leukocyte rolling velocity in postcapillary venules of the rat mesentery over 1 h. 3. Likewise, aminoguanidine (10-100 mg kg-1, i.v.) dose-dependently increased leukocyte adherence and decreased leukocyte rolling velocity over 1 h. 4. Both L-NAME and aminoguanidine caused a dose-dependent reduction in mesenteric arteriolar diameter and an increase in systemic arterial blood pressure. 5. The effects of aminoguanidine (50 mg kg-1, i.v.) on leukocyte adherence, arteriolar diameter and on blood pressure were significantly reversed by pretreatment with L-arginine (300 mg kg-1, i.v.). 6. These findings indicate that, like L-NAME, aminoguanidine can acutely promote leukocyte adherence to the mesenteric venular wall and reduce arteriolar diameter. Moreover, these acute effects were reversed by L-arginine, suggesting they are mediated through inhibition of constitutive NO synthase.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arndt H., Russell J. B., Kurose I., Kubes P., Granger D. N. Mediators of leukocyte adhesion in rat mesenteric venules elicited by inhibition of nitric oxide synthesis. Gastroenterology. 1993 Sep;105(3):675–680. doi: 10.1016/0016-5085(93)90882-d. [DOI] [PubMed] [Google Scholar]
- Bath P. M., Hassall D. G., Gladwin A. M., Palmer R. M., Martin J. F. Nitric oxide and prostacyclin. Divergence of inhibitory effects on monocyte chemotaxis and adhesion to endothelium in vitro. Arterioscler Thromb. 1991 Mar-Apr;11(2):254–260. doi: 10.1161/01.atv.11.2.254. [DOI] [PubMed] [Google Scholar]
- Boughton-Smith N. K., Evans S. M., Laszlo F., Whittle B. J., Moncada S. The induction of nitric oxide synthase and intestinal vascular permeability by endotoxin in the rat. Br J Pharmacol. 1993 Nov;110(3):1189–1195. doi: 10.1111/j.1476-5381.1993.tb13940.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardinal D. C., Higgs G. A. A photometric device for measuring blood vessel diameter in the microcirculation. J Pharmacol Methods. 1980 Sep;4(2):109–114. doi: 10.1016/0160-5402(80)90030-3. [DOI] [PubMed] [Google Scholar]
- Corbett J. A., Tilton R. G., Chang K., Hasan K. S., Ido Y., Wang J. L., Sweetland M. A., Lancaster J. R., Jr, Williamson J. R., McDaniel M. L. Aminoguanidine, a novel inhibitor of nitric oxide formation, prevents diabetic vascular dysfunction. Diabetes. 1992 Apr;41(4):552–556. doi: 10.2337/diab.41.4.552. [DOI] [PubMed] [Google Scholar]
- Griffiths M. J., Messent M., MacAllister R. J., Evans T. W. Aminoguanidine selectively inhibits inducible nitric oxide synthase. Br J Pharmacol. 1993 Nov;110(3):963–968. doi: 10.1111/j.1476-5381.1993.tb13907.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan K., Heesen B. J., Corbett J. A., McDaniel M. L., Chang K., Allison W., Wolffenbuttel B. H., Williamson J. R., Tilton R. G. Inhibition of nitric oxide formation by guanidines. Eur J Pharmacol. 1993 Nov 2;249(1):101–106. doi: 10.1016/0014-2999(93)90667-7. [DOI] [PubMed] [Google Scholar]
- Joly G. A., Ayres M., Chelly F., Kilbourn R. G. Effects of NG-methyl-L-arginine, NG-nitro-L-arginine, and aminoguanidine on constitutive and inducible nitric oxide synthase in rat aorta. Biochem Biophys Res Commun. 1994 Feb 28;199(1):147–154. doi: 10.1006/bbrc.1994.1207. [DOI] [PubMed] [Google Scholar]
- Kubes P., Kanwar S., Niu X. F., Gaboury J. P. Nitric oxide synthesis inhibition induces leukocyte adhesion via superoxide and mast cells. FASEB J. 1993 Oct;7(13):1293–1299. doi: 10.1096/fasebj.7.13.8405815. [DOI] [PubMed] [Google Scholar]
- Kubes P., Suzuki M., Granger D. N. Nitric oxide: an endogenous modulator of leukocyte adhesion. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):4651–4655. doi: 10.1073/pnas.88.11.4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laszlo F., Evans S. M., Whittle B. J. Aminoguanidine inhibits both constitutive and inducible nitric oxide synthase isoforms in rat intestinal microvasculature in vivo. Eur J Pharmacol. 1995 Jan 16;272(2-3):169–175. doi: 10.1016/0014-2999(94)00637-m. [DOI] [PubMed] [Google Scholar]
- Laszlo F., Whittle B. J., Moncada S. Time-dependent enhancement or inhibition of endotoxin-induced vascular injury in rat intestine by nitric oxide synthase inhibitors. Br J Pharmacol. 1994 Apr;111(4):1309–1315. doi: 10.1111/j.1476-5381.1994.tb14887.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence M. B., Springer T. A. Leukocytes roll on a selectin at physiologic flow rates: distinction from and prerequisite for adhesion through integrins. Cell. 1991 May 31;65(5):859–873. doi: 10.1016/0092-8674(91)90393-d. [DOI] [PubMed] [Google Scholar]
- László F., Whittle B. J., Moncada S. Attenuation by nitrosothiol NO donors of acute intestinal microvascular dysfunction in the rat. Br J Pharmacol. 1995 Jun;115(3):498–502. doi: 10.1111/j.1476-5381.1995.tb16361.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- László F., Whittle B. J., Moncada S. Interactions of constitutive nitric oxide with PAF and thromboxane on rat intestinal vascular integrity in acute endotoxaemia. Br J Pharmacol. 1994 Dec;113(4):1131–1136. doi: 10.1111/j.1476-5381.1994.tb17114.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misko T. P., Moore W. M., Kasten T. P., Nickols G. A., Corbett J. A., Tilton R. G., McDaniel M. L., Williamson J. R., Currie M. G. Selective inhibition of the inducible nitric oxide synthase by aminoguanidine. Eur J Pharmacol. 1993 Mar 16;233(1):119–125. doi: 10.1016/0014-2999(93)90357-n. [DOI] [PubMed] [Google Scholar]
- Moncada S., Palmer R. M., Higgs E. A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991 Jun;43(2):109–142. [PubMed] [Google Scholar]
- Ohrui T., Yamauchi K., Sekizawa K., Ohkawara Y., Maeyama K., Sasaki M., Takemura M., Wada H., Watanabe T., Sasaki H. Histamine N-methyltransferase controls the contractile response of guinea pig trachea to histamine. J Pharmacol Exp Ther. 1992 Jun;261(3):1268–1272. [PubMed] [Google Scholar]
- Rees D. D., Palmer R. M., Moncada S. Role of endothelium-derived nitric oxide in the regulation of blood pressure. Proc Natl Acad Sci U S A. 1989 May;86(9):3375–3378. doi: 10.1073/pnas.86.9.3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees D. D., Palmer R. M., Schulz R., Hodson H. F., Moncada S. Characterization of three inhibitors of endothelial nitric oxide synthase in vitro and in vivo. Br J Pharmacol. 1990 Nov;101(3):746–752. doi: 10.1111/j.1476-5381.1990.tb14151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salter M., Knowles R. G., Moncada S. Widespread tissue distribution, species distribution and changes in activity of Ca(2+)-dependent and Ca(2+)-independent nitric oxide synthases. FEBS Lett. 1991 Oct 7;291(1):145–149. doi: 10.1016/0014-5793(91)81123-p. [DOI] [PubMed] [Google Scholar]
- Whittle B. J., Lopez-Belmonte J., Rees D. D. Modulation of the vasodepressor actions of acetylcholine, bradykinin, substance P and endothelin in the rat by a specific inhibitor of nitric oxide formation. Br J Pharmacol. 1989 Oct;98(2):646–652. doi: 10.1111/j.1476-5381.1989.tb12639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


