Abstract
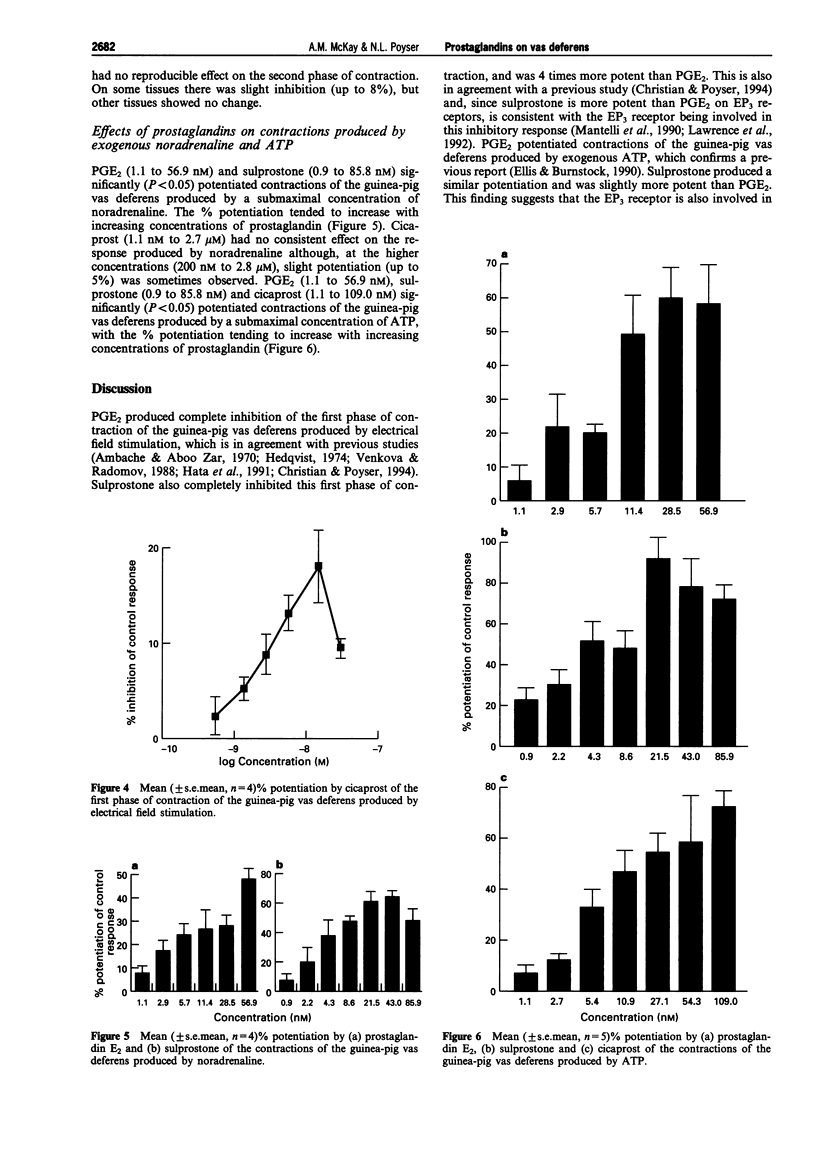
1. This study has compared the effects of exogenous and endogenous prostaglandins on the two phases of contraction of the guinea-pig vas deferens produced by electrical field stimulation. Prostaglandin E2 (PGE2), sulprostone and arachidonic acid dose-dependently and completely inhibited the first (fast) phase of contraction, with IC50s of 2.6 nM, 0.65 nM and 2.2 microM, respectively. 2. Following desensitization of the receptor for adenosine triphosphate (ATP) with alpha, beta-methylene ATP, PGE2, sulprostone and arachidonic acid dose-dependently inhibited the second (slow) phase of contraction of the guinea-pig vas deferens produced by electrical field stimulation, but the inhibition was incomplete (up to only 30%). Indomethacin (2.8 microM) reduced the effect of arachidonic acid. On its own, indomethacin (0.3 to 6.0 microM) had no consistent effect although, on some tissues, a slight potentiation of the contractions was seen. 3. Cicaprost (a PGI2 analogue) at low concentrations (0.5 to 30 nM) potentiated the first phase of contraction but even at high concentrations, had no consistent effect on the second phase of contraction of the guinea-pig vas deferens produced by electrical field stimulation. 4. PGE2, sulprostone and cicaprost potentiated contractions of the guinea-pig vas deferens produced by exogenous ATP. PGE2 and sulprostone also potentiated contractions produced by exogenous noradrenaline, whereas cicaprost had no consistent effect on the response to noradrenaline. 5. These findings indicate that prostaglandins of the E-series inhibit the second phase of contraction as well as the first phase of contraction of the guinea-pig vas deferens produced by electrical field stimulation. However, the extent of the inhibition is much less for the second phase than for the first phase. The reasons for this differential action of PGE are not clear. 6. Cicaprost potentiates the first phase but not the second phase of contraction. Since cicaprost potentiates the contractions produced by exogenous ATP, but not by exogenous noradrenaline, by an action presumably on post-junctional IP receptors, the potentiating action of cicaprost on the first phase of contraction produced by electrical field stimulation would appear to be satisfactorily explained through the action of cicaprost on these post-junctional IP receptors. 7. Exogenous arachidonic acid is apparently converted predominantly to PGE2 by the vas deferens, since the action of arachidonic acid mimicked that of PGE2 and was reduced by indomethacin. However, there was little evidence that sufficient PGE2 is generated during a short period (15 s) of sympathetic nerve stimulation for it to have any significant inhibitory effect on the size of the contractions produced.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambache N., Zar M. A. An inhibitory effect of prostaglandin E2 on neuromuscular transmission in the guinea-pig vas deferens. J Physiol. 1970 May;208(1):30P–32P. [PubMed] [Google Scholar]
- Bedwani J. R., Blanning P. E. Effects of prostaglandin E2 on fast and slow components of the response of the rat vas deferens to field stimulation. Br J Pharmacol. 1983 Jan;78(1):143–150. doi: 10.1111/j.1476-5381.1983.tb09374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G. Overview. Purinergic mechanisms. Ann N Y Acad Sci. 1990;603:1–18. doi: 10.1111/j.1749-6632.1990.tb37657.x. [DOI] [PubMed] [Google Scholar]
- Christian H. C., Poyser N. L. Effects of exogenous and endogenous prostaglandins on the fast phase of contraction of the guinea-pig vas deferens produced by electrical field stimulation. Prostaglandins Leukot Essent Fatty Acids. 1994 Jul;51(1):57–62. doi: 10.1016/0952-3278(94)90179-1. [DOI] [PubMed] [Google Scholar]
- Driessen B., Starke K. Modulation of neural noradrenaline and ATP release by angiotensin II and prostaglandin E2 in guinea-pig vas deferens. Naunyn Schmiedebergs Arch Pharmacol. 1994 Dec;350(6):618–625. doi: 10.1007/BF00169366. [DOI] [PubMed] [Google Scholar]
- Ellis J. L., Burnstock G. Modulation by prostaglandin E2 of ATP and noradrenaline co-transmission in the guinea-pig vas deferens. J Auton Pharmacol. 1990 Dec;10(6):363–372. doi: 10.1111/j.1474-8673.1990.tb00036.x. [DOI] [PubMed] [Google Scholar]
- Fried G., Lagercrantz H., Hökfelt T. Improved isolation of small noradrenergic vesicles from rat seminal ducts following castration. A density gradient centrifugation and morphological study. Neuroscience. 1978;3(12):1271–1291. doi: 10.1016/0306-4522(78)90147-1. [DOI] [PubMed] [Google Scholar]
- Fujita A., Takeuchi T., Hata F., Yagasaki O. Role of PGE2 in neurotransmission from pre- to post-ganglionic hypogastric nerves of guinea pigs. Jpn J Pharmacol. 1992 Jan;58(1):61–66. doi: 10.1254/jjp.58.61. [DOI] [PubMed] [Google Scholar]
- Hata F., Kishi I., Saeki K., Takeuchi T., Yagasaki O. Prostaglandin E2 selectively affects purinergic transmission in guinea pig vas deferens. Neuropharmacology. 1991 Oct;30(10):1107–1112. doi: 10.1016/0028-3908(91)90140-7. [DOI] [PubMed] [Google Scholar]
- Hedqvist P. Basic mechanisms of prostaglandin action on autonomic neurotransmission. Annu Rev Pharmacol Toxicol. 1977;17:259–279. doi: 10.1146/annurev.pa.17.040177.001355. [DOI] [PubMed] [Google Scholar]
- Hedqvist P. Prostaglandin action on noradrenaline release and mechanical responses in the stimulated guinea pig vas deferens. Acta Physiol Scand. 1974 Jan;90(1):86–93. doi: 10.1111/j.1748-1716.1974.tb05566.x. [DOI] [PubMed] [Google Scholar]
- Hedqvist P., von Euler U. S. Prostaglandin controls neuromuscular transmission in guinea-pig vas deferens. Nat New Biol. 1972 Mar 29;236(65):113–115. doi: 10.1038/newbio236113a0. [DOI] [PubMed] [Google Scholar]
- Ikeda S. R. Prostaglandin modulation of Ca2+ channels in rat sympathetic neurones is mediated by guanine nucleotide binding proteins. J Physiol. 1992 Dec;458:339–359. doi: 10.1113/jphysiol.1992.sp019421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagercrantz H. On the composition and function of large dense cored vesicles in sympathetic nerves. Neuroscience. 1976;1(2):81–92. doi: 10.1016/0306-4522(76)90002-6. [DOI] [PubMed] [Google Scholar]
- Lawrence R. A., Jones R. L., Wilson N. H. Characterization of receptors involved in the direct and indirect actions of prostaglandins E and I on the guinea-pig ileum. Br J Pharmacol. 1992 Feb;105(2):271–278. doi: 10.1111/j.1476-5381.1992.tb14245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew M. J., White T. D. Release of endogenous ATP during sympathetic nerve stimulation. Br J Pharmacol. 1987 Oct;92(2):349–355. doi: 10.1111/j.1476-5381.1987.tb11330.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantelli L., Amerini S., Rubino A., Ledda F. Prejunctional prostanoid receptors on cardiac adrenergic terminals belong to the EP3 subtype. Br J Pharmacol. 1991 Mar;102(3):573–576. doi: 10.1111/j.1476-5381.1991.tb12214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mo N., Ammari R., Dun N. J. Prostaglandin E1 inhibits calcium-dependent potentials in mammalian sympathetic neurons. Brain Res. 1985 May 20;334(2):325–329. doi: 10.1016/0006-8993(85)90225-2. [DOI] [PubMed] [Google Scholar]
- Stjärne L., Astrand P. Relative pre- and postjunctional roles of noradrenaline and adenosine 5'-triphosphate as neurotransmitters of the sympathetic nerves of guinea-pig and mouse vas deferens. Neuroscience. 1985 Mar;14(3):929–946. doi: 10.1016/0306-4522(85)90155-1. [DOI] [PubMed] [Google Scholar]
- Stjärne L. Prostaglandin E restricting noradrenaline secretion--neural in origin? Acta Physiol Scand. 1972 Dec;86(4):574–576. doi: 10.1111/j.1748-1716.1972.tb05360.x. [DOI] [PubMed] [Google Scholar]
- Sugimoto Y., Namba T., Honda A., Hayashi Y., Negishi M., Ichikawa A., Narumiya S. Cloning and expression of a cDNA for mouse prostaglandin E receptor EP3 subtype. J Biol Chem. 1992 Apr 5;267(10):6463–6466. [PubMed] [Google Scholar]
- Sugimoto Y., Negishi M., Hayashi Y., Namba T., Honda A., Watabe A., Hirata M., Narumiya S., Ichikawa A. Two isoforms of the EP3 receptor with different carboxyl-terminal domains. Identical ligand binding properties and different coupling properties with Gi proteins. J Biol Chem. 1993 Feb 5;268(4):2712–2718. [PubMed] [Google Scholar]
- Swan C. G., Poyser N. L. Prostaglandin synthesis by, and the effects of prostaglandins and prostaglandin analogues on, the vas deferens of the rabbit and rat in vitro. J Reprod Fertil. 1983 Sep;69(1):91–99. doi: 10.1530/jrf.0.0690091. [DOI] [PubMed] [Google Scholar]
- Swedin G. Biphasic mechanical response of the isolated vas deferens to nerve stimulation. Acta Physiol Scand. 1971 Apr;81(4):574–576. doi: 10.1111/j.1748-1716.1971.tb04936.x. [DOI] [PubMed] [Google Scholar]
- Trachte G. J. The influence of prostaglandins on neurotransmission in the rabbit isolated vas deferens. Prostaglandins. 1985 Jan;29(1):47–59. doi: 10.1016/0090-6980(85)90150-9. [DOI] [PubMed] [Google Scholar]
- Venkova K., Radomirov R. Contractile responses of isolated guinea-pig vas deferens to trains of electrical stimuli and influence of prostaglandin E2. Pharmacol Res Commun. 1988 Apr;20(4):277–292. doi: 10.1016/s0031-6989(88)80065-1. [DOI] [PubMed] [Google Scholar]
- White T. D., MacDonald W. F. Neural release of ATP and adenosine. Ann N Y Acad Sci. 1990;603:287–299. doi: 10.1111/j.1749-6632.1990.tb37680.x. [DOI] [PubMed] [Google Scholar]
- von Kügelgen I., Starke K. Release of noradrenaline and ATP by electrical stimulation and nicotine in guinea-pig vas deferens. Naunyn Schmiedebergs Arch Pharmacol. 1991 Oct;344(4):419–429. doi: 10.1007/BF00172581. [DOI] [PubMed] [Google Scholar]



