Abstract
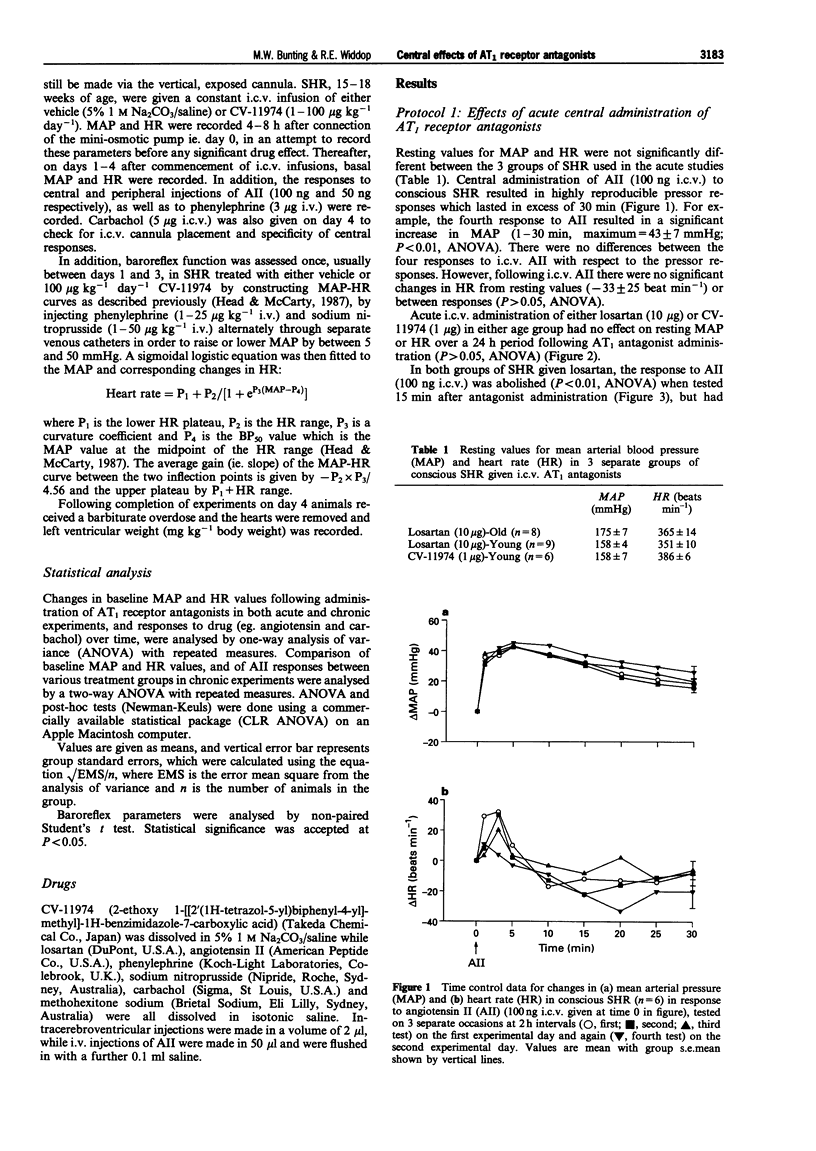
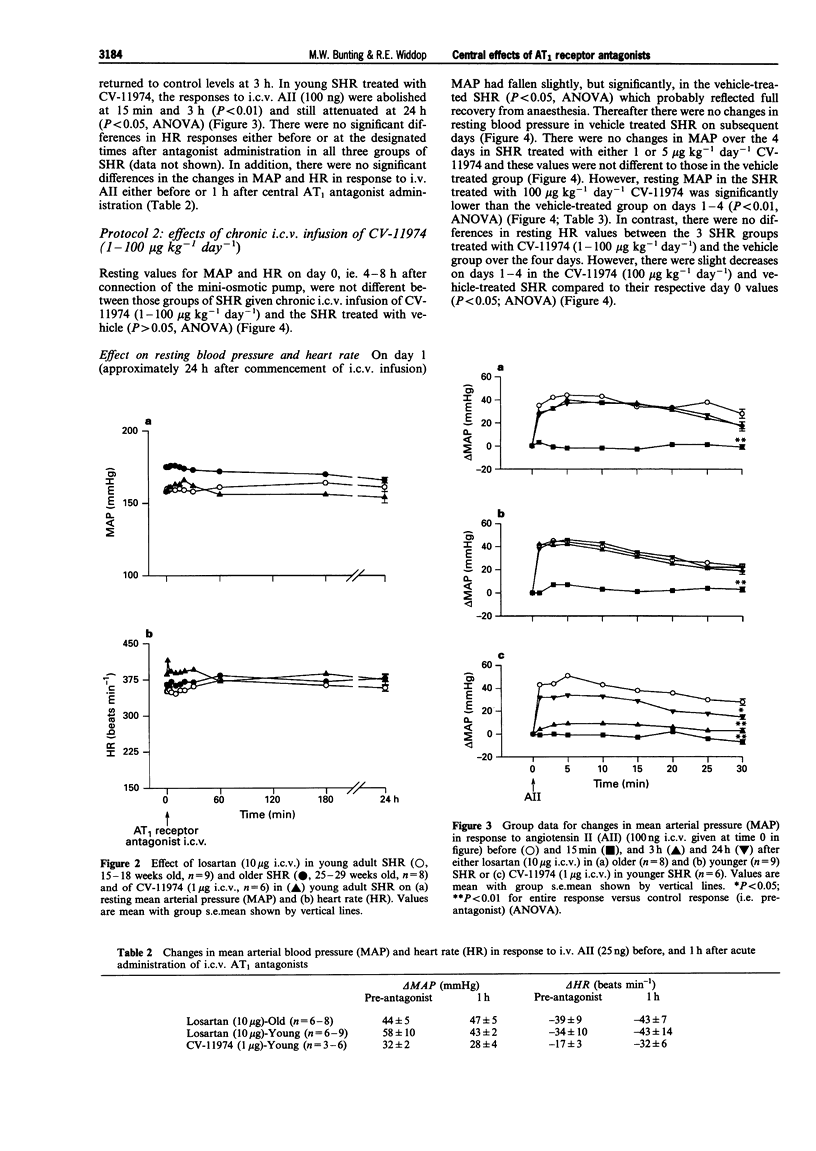
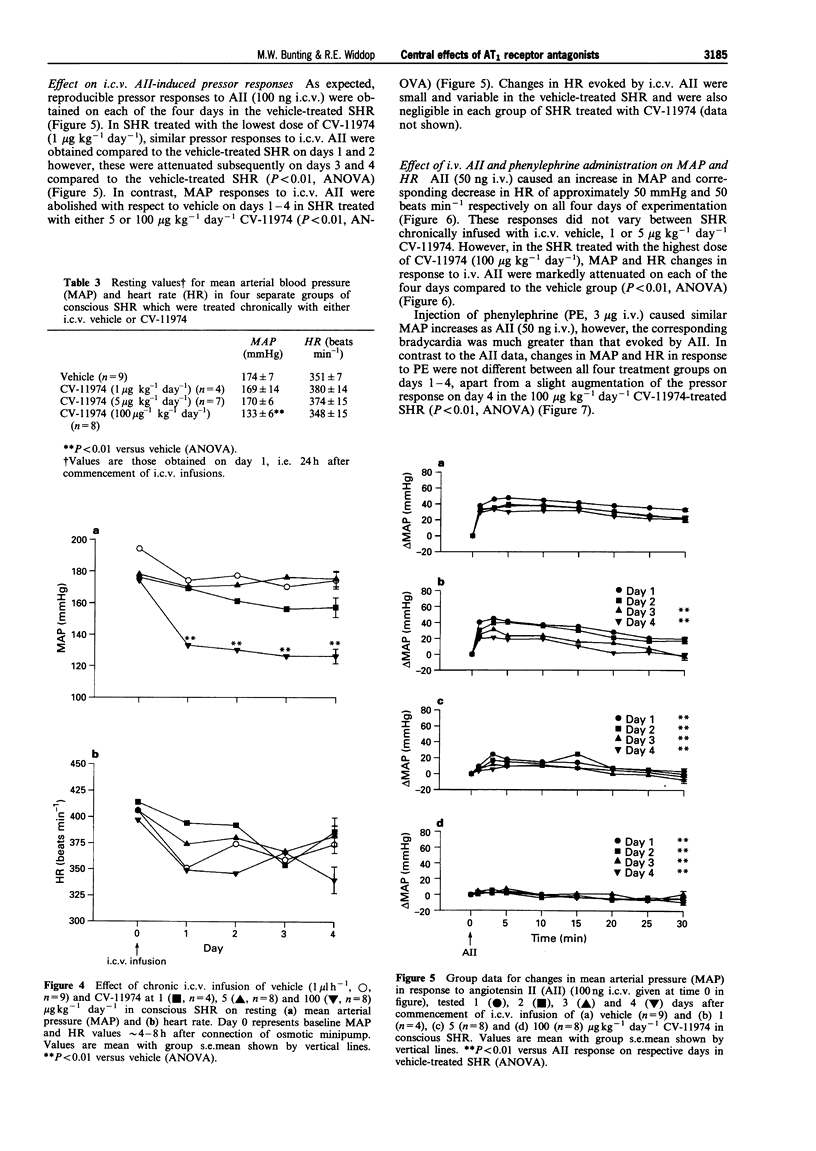
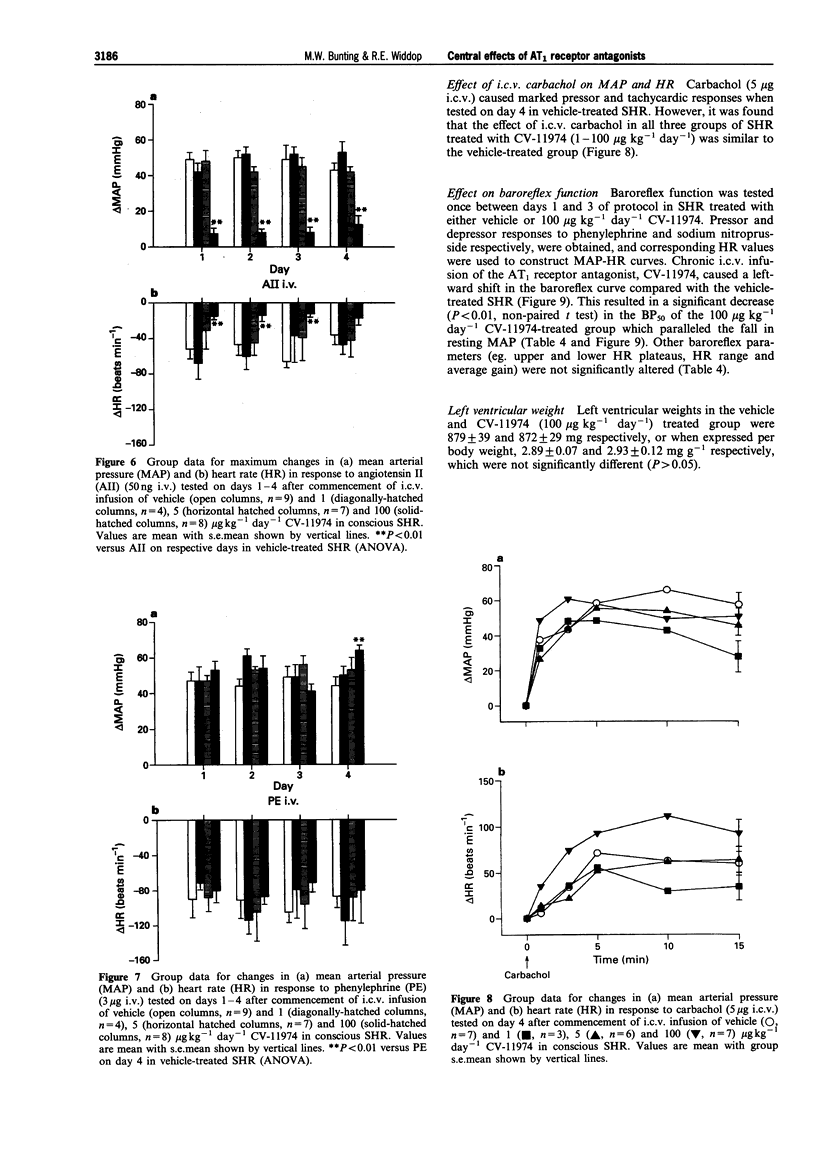
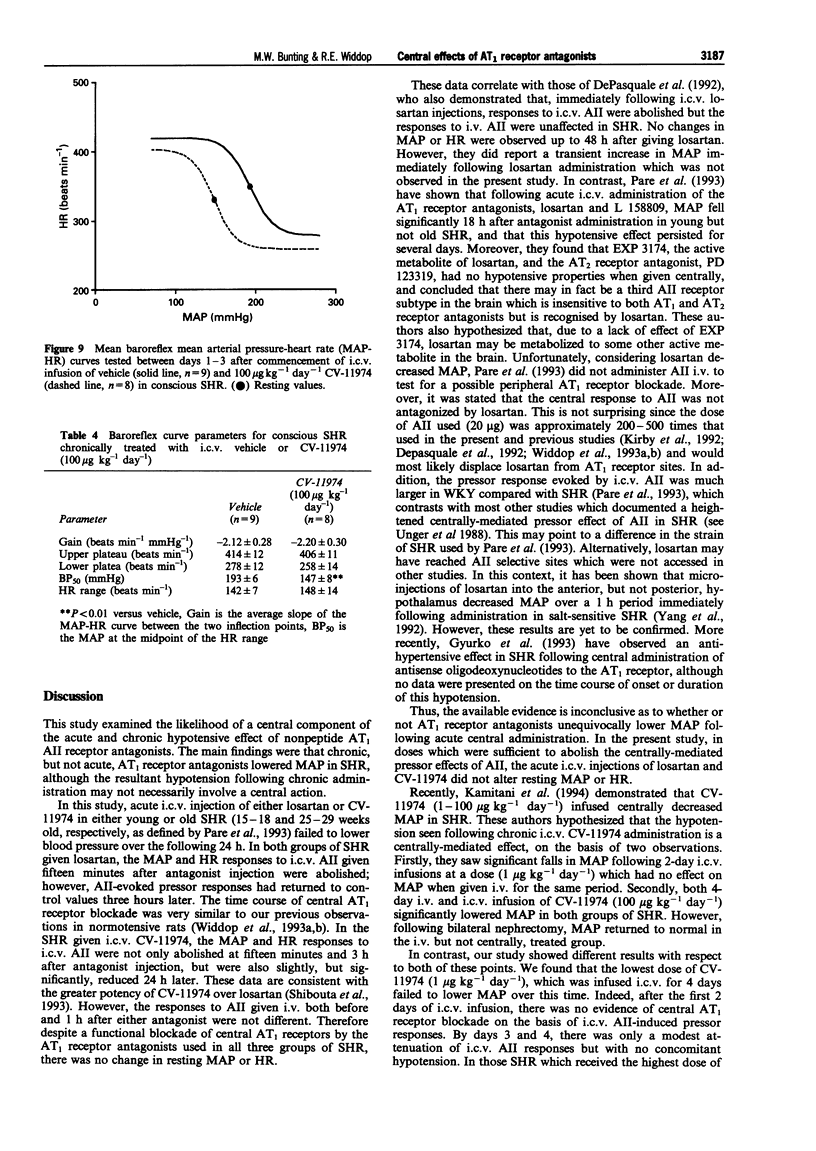
1. The role of the central renin-angiotensin system in the pathogenesis of hypertension in spontaneously hypertensive rats (SHR) was examined following acute and chronic intracerebroventricular (i.c.v.) infusions of angiotensin1 (AT1) receptor antagonists. 2. Groups of SHR were chronically instrumented for acute i.c.v. administration of the AT1 receptor antagonists, losartan and CV-11974, on mean arterial blood pressure (MAP) and heart rate (HR). Other groups of SHR also had mini-osmotic pumps implanted for chronic i.c.v. infusion of CV-11974. 3. Initially both young (15-18 weeks, n = 8) and old (25-29 weeks, n = 9) SHR received acute i.c.v. injections of losartan (10 micrograms) while a third group of young SHR received CV-11974 (1 microgram, n = 6). In all three groups of SHR, MAP and HR did not change up to 24 h after antagonist injection. However, changes in MAP and HR in response to i.c.v. angiotensin II (AII, 100 ng) were abolished 15 min after administration of the AT1 receptor antagonists. These responses had returned to control levels after 3 h in both groups given losartan but were still significantly depressed at 24 h in the CV-11974-treated group. By contrast, responses to i.v. AII (25 ng) before and 1 h after administration of AT1 receptor antagonists were not significantly different. 4. For chronic studies, four groups of SHR received chronic i.c.v. infusion of either vehicle (n = 9) or CV-11974 (1, 5 and 100 micrograms kg-1 day-1) (n = 4, 7 and 8 respectively) for 4 days. Baseline cardiovascular parameters were monitored daily together with changes in MAP and HR in response to both i.c.v. and i.v. AII (100 ng and 50 ng respectively) and i.v. phenylephrine (3 micrograms). Responses to i.c.v. carbachol (5 micrograms) were also recorded on day 4 while baroreflex function was assessed between days 1-3. In SHR treated chronically with i.c.v. vehicle or CV-11974, at 1 or 5 micrograms kg-1 day-1, resting MAP and HR did not vary over the four day infusion period. However, SHR treated with 100 micrograms kg-1 day-1 CV-11974 had significantly lower MAP compared to vehicle-treated SHR. While there was some variation in resting HR, there were no differences between the drug-treated and vehicle-treated groups. Pressor responses following i.c.v. AII administration were slightly, but significantly, inhibited on days 3 and 4 in the low dose CV-11974-treated (1 microgram kg-1 day-1) SHR. However, these responses were abolished on all 4 days in the 5 and 100 micrograms kg-1 day-1 CV-11974-treated groups. By contrast, changes in MAP and HR following i.v. AII injection did not vary over the 4 day infusion between SHR treated with the 2 lowest doses of CV-11974 and the vehicle-treated group. However, in the high dose CV-11974-treated SHR (100 micrograms kg-1 day-1), the cardiovascular effects of AII were abolished. In addition, phenylephrine (i.v.) and carbachol (i.c.v.) induced changes in MAP and HR were not significantly different in all four treatment groups. Similarly, baroreflex function was unaffected by i.c.v. infusion of 100 micrograms kg-1 day-1 CV-11974, except for a significant fall in BP50 which paralleled the fall in resting MAP. 5. Collectively, these results indicate that acute and chronic central AT1 receptor antagonism does not lower MAP in conscious SHR in doses which only block central AII-induced pressor activity. Chronic central infusion of CV-11974 at sufficiently high doses will lower MAP, as has been reported by others, but not without the abolition of the peripheral effects of AII. Therefore it is most likely that peripheral AT1 receptor blockade contributes to the hypotensive action of CV-11974 under these conditions.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartholomeusz B., Widdop R. E. Effect of acute and chronic treatment with the angiotensin II subtype 1 receptor antagonist EXP 3174 on baroreflex function in conscious spontaneously hypertensive rats. J Hypertens. 1995 Feb;13(2):219–225. [PubMed] [Google Scholar]
- Berecek K. H., Robertson J. D., Thorstad M. H. Central administration of a specific angiotensin II receptor antagonist on baroreflex function in spontaneously hypertensive rats. J Hypertens. 1991 Apr;9(4):365–371. doi: 10.1097/00004872-199104000-00009. [DOI] [PubMed] [Google Scholar]
- Beresford M. J., Fitzsimons J. T. Intracerebroventricular angiotensin II-induced thirst and sodium appetite in rat are blocked by the AT1 receptor antagonist, Losartan (DuP 753), but not by the AT2 antagonist, CGP 42112B. Exp Physiol. 1992 Sep;77(5):761–764. doi: 10.1113/expphysiol.1992.sp003643. [DOI] [PubMed] [Google Scholar]
- Bruner C. A., Kuslikis B. I., Fink G. D. Effect of inhibition of central angiotensin pressor mechanisms on blood pressure in spontaneously hypertensive rats. J Cardiovasc Pharmacol. 1987 Mar;9(3):298–304. doi: 10.1097/00005344-198703000-00006. [DOI] [PubMed] [Google Scholar]
- Buñag R. D., Eriksson L., Tanabe S. Baroreceptor reflex enhancement by chronic intracerebroventricular infusion of enalapril in normotensive rats. Hypertension. 1990 Mar;15(3):284–290. doi: 10.1161/01.hyp.15.3.284. [DOI] [PubMed] [Google Scholar]
- DePasquale M. J., Fossa A. A., Holt W. F., Mangiapane M. L. Central DuP 753 does not lower blood pressure in spontaneously hypertensive rats. Hypertension. 1992 Jun;19(6 Pt 2):668–671. doi: 10.1161/01.hyp.19.6.668. [DOI] [PubMed] [Google Scholar]
- Edmunds M. E., Russell G. I., Burton P. R., Swales J. D. Baroreceptor-heart rate reflex function before and after surgical reversal of two-kidney, one-clip hypertension in the rat. Circ Res. 1990 Jun;66(6):1673–1680. doi: 10.1161/01.res.66.6.1673. [DOI] [PubMed] [Google Scholar]
- Gruber K. A., Callahan M. F., Eskridge-Sloop S. L. Central administration of angiotensin II receptor antagonists and arterial pressure regulation: a note of caution. Life Sci. 1992;50(20):1497–1502. doi: 10.1016/0024-3205(92)90139-g. [DOI] [PubMed] [Google Scholar]
- Gyurko R., Wielbo D., Phillips M. I. Antisense inhibition of AT1 receptor mRNA and angiotensinogen mRNA in the brain of spontaneously hypertensive rats reduces hypertension of neurogenic origin. Regul Pept. 1993 Dec 10;49(2):167–174. doi: 10.1016/0167-0115(93)90438-e. [DOI] [PubMed] [Google Scholar]
- Head G. A., McCarty R. Vagal and sympathetic components of the heart rate range and gain of the baroreceptor-heart rate reflex in conscious rats. J Auton Nerv Syst. 1987 Dec;21(2-3):203–213. doi: 10.1016/0165-1838(87)90023-3. [DOI] [PubMed] [Google Scholar]
- Hogarty D. C., Speakman E. A., Puig V., Phillips M. I. The role of angiotensin, AT1 and AT2 receptors in the pressor, drinking and vasopressin responses to central angiotensin. Brain Res. 1992 Jul 24;586(2):289–294. doi: 10.1016/0006-8993(92)91638-u. [DOI] [PubMed] [Google Scholar]
- Jensen L. L., Harding J. W., Wright J. W. Central effects of a specific angiotensin receptor antagonist, sarthran (Sar1, Thr8AII) in normotensive and spontaneously hypertensive rat strains. Brain Res. 1988 May 17;448(2):359–363. doi: 10.1016/0006-8993(88)91277-2. [DOI] [PubMed] [Google Scholar]
- Kamitani A., Higashimori K., Kohara K., Higaki J., Mikami H., Ogihara T. The effects of central administration of angiotensin II type-1 receptor antagonist, CV-11974, in nephrectomized spontaneously hypertensive rats. Clin Exp Pharmacol Physiol. 1994 Apr;21(4):271–276. doi: 10.1111/j.1440-1681.1994.tb02512.x. [DOI] [PubMed] [Google Scholar]
- Kirby R. F., Thunhorst R. L., Johnson A. K. Effects of a non-peptide angiotensin receptor antagonist on drinking and blood pressure responses to centrally administered angiotensins in the rat. Brain Res. 1992 Apr 3;576(2):348–350. doi: 10.1016/0006-8993(92)90703-c. [DOI] [PubMed] [Google Scholar]
- Mann J. F., Phillips M. I., Dietz R., Haebara H., Ganten D. Effects of central and peripheral angiotensin blockade in hypertensive rats. Am J Physiol. 1978 May;234(5):H629–H637. doi: 10.1152/ajpheart.1978.234.5.H629. [DOI] [PubMed] [Google Scholar]
- McDonald W., Wickre C., Aumann S., Ban D., Moffitt B. The sustained antihypertensive effect of chronic cerebroventricular infusion of angiotensin antagonist in spontaneously hypertensive rats. Endocrinology. 1980 Nov;107(5):1305–1308. doi: 10.1210/endo-107-5-1305. [DOI] [PubMed] [Google Scholar]
- Minami N., Head G. A. Relationship between cardiovascular hypertrophy and cardiac baroreflex function in spontaneously hypertensive and stroke-prone rats. J Hypertens. 1993 May;11(5):523–533. doi: 10.1097/00004872-199305000-00008. [DOI] [PubMed] [Google Scholar]
- Okuno T., Nagahama S., Lindheimer M. D., Oparil S. Attenuation of the development of spontaneous hypertension in rats by chronic central administration of captopril. Hypertension. 1983 Sep-Oct;5(5):653–662. doi: 10.1161/01.hyp.5.5.653. [DOI] [PubMed] [Google Scholar]
- Paré M. C., Maltais S., Escher E. The neurogenic origin of hypertension in SHR may be mediated by angiotensin II through a receptor different from AT1 and AT2. Regul Pept. 1993 Aug 13;47(1):81–86. doi: 10.1016/0167-0115(93)90275-d. [DOI] [PubMed] [Google Scholar]
- Phillips M. I., Kimura B. Converting enzyme inhibitors and brain angiotensin. J Cardiovasc Pharmacol. 1986;8 (Suppl 10):S82–S90. [PubMed] [Google Scholar]
- Phillips M. I., Mann J. F., Haebara H., Hoffman W. E., Dietz R., Schelling P., Ganten D. Lowering of hypertension by central saralasin in the absence of plasma renin. Nature. 1977 Dec 1;270(5636):445–447. doi: 10.1038/270445a0. [DOI] [PubMed] [Google Scholar]
- Reid I. A. Actions of angiotensin II on the brain: mechanisms and physiologic role. Am J Physiol. 1984 May;246(5 Pt 2):F533–F543. doi: 10.1152/ajprenal.1984.246.5.F533. [DOI] [PubMed] [Google Scholar]
- Shibouta Y., Inada Y., Ojima M., Wada T., Noda M., Sanada T., Kubo K., Kohara Y., Naka T., Nishikawa K. Pharmacological profile of a highly potent and long-acting angiotensin II receptor antagonist, 2-ethoxy-1-[[2'-(1H-tetrazol-5-yl)biphenyl-4- yl]methyl]-1H-benzimidazole-7-carboxylic acid (CV-11974), and its prodrug, (+/-)-1-(cyclohexyloxycarbonyloxy)-ethyl 2-ethoxy-1-[[2'-(1H-tetrazol-5- yl)biphenyl-4-yl]methyl]-1H-benzimidazole-7-carboxylate (TCV-116). J Pharmacol Exp Ther. 1993 Jul;266(1):114–120. [PubMed] [Google Scholar]
- Stamler J. F., Raizada M. K., Fellows R. E., Phillips M. I. Increased specific binding of angiotensin II in the organum vasculosum of the laminae terminalis area of the spontaneously hypertensive rat brain. Neurosci Lett. 1980 Apr;17(1-2):173–177. doi: 10.1016/0304-3940(80)90080-4. [DOI] [PubMed] [Google Scholar]
- Steckelings U., Lebrun C., Qadri F., Veltmar A., Unger T. Role of brain angiotensin in cardiovascular regulation. J Cardiovasc Pharmacol. 1992;19 (Suppl 6):S72–S79. doi: 10.1097/00005344-199219006-00012. [DOI] [PubMed] [Google Scholar]
- Sweet C. S., Columbo J. M., Gaul S. L. Central antihypertensive effects of inhibitors of the renin-angiotensin system in rats. Am J Physiol. 1976 Dec;231(6):1794–1799. doi: 10.1152/ajplegacy.1976.231.6.1794. [DOI] [PubMed] [Google Scholar]
- Timmermans P. B., Wong P. C., Chiu A. T., Herblin W. F., Benfield P., Carini D. J., Lee R. J., Wexler R. R., Saye J. A., Smith R. D. Angiotensin II receptors and angiotensin II receptor antagonists. Pharmacol Rev. 1993 Jun;45(2):205–251. [PubMed] [Google Scholar]
- Toney G. M., Porter J. P. Functional role of brain AT1 and AT2 receptors in the central angiotensin II pressor response. Brain Res. 1993 Feb 12;603(1):57–63. doi: 10.1016/0006-8993(93)91299-8. [DOI] [PubMed] [Google Scholar]
- Toney G. M., Porter J. P. Functional roles of brain AT1 and AT2 receptors in the central angiotensin II pressor response in conscious young spontaneously hypertensive rats. Brain Res Dev Brain Res. 1993 Feb 19;71(2):193–199. doi: 10.1016/0165-3806(93)90171-6. [DOI] [PubMed] [Google Scholar]
- Trippodo N. C., Frohlich E. D. Similarities of genetic (spontaneous) hypertension. Man and rat. Circ Res. 1981 Mar;48(3):309–319. doi: 10.1161/01.res.48.3.309. [DOI] [PubMed] [Google Scholar]
- Unger T., Badoer E., Ganten D., Lang R. E., Rettig R. Brain angiotensin: pathways and pharmacology. Circulation. 1988 Jun;77(6 Pt 2):I40–I54. [PubMed] [Google Scholar]
- Unger T., Rascher W., Schuster C., Pavlovitch R., Schömig A., Dietz R., Ganten D. Central blood pressure effects of substance P and angiotensin II: role of the sympathetic nervous system and vasopressin. Eur J Pharmacol. 1981 Apr 24;71(1):33–42. doi: 10.1016/0014-2999(81)90384-8. [DOI] [PubMed] [Google Scholar]
- Widdop R. E., Gardiner S. M., Kemp P. A., Bennett T. Central administration of PD 123319 or EXP-3174 inhibits effects of angiotensin II. Am J Physiol. 1993 Jan;264(1 Pt 2):H117–H125. doi: 10.1152/ajpheart.1993.264.1.H117. [DOI] [PubMed] [Google Scholar]
- Widdop R. E., Gardiner S. M., Kemp P. A., Bennett T. Differential blockade of central effects of angiotensin II by AT2-receptor antagonists. Am J Physiol. 1993 Jul;265(1 Pt 2):H226–H231. doi: 10.1152/ajpheart.1993.265.1.H226. [DOI] [PubMed] [Google Scholar]
- Widdop R. E., Gardiner S. M., Kemp P. A., Bennett T. Inhibition of the haemodynamic effects of angiotensin II in conscious rats by AT2-receptor antagonists given after the AT1-receptor antagonist, EXP 3174. Br J Pharmacol. 1992 Nov;107(3):873–880. doi: 10.1111/j.1476-5381.1992.tb14540.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong P. C., Price W. A., Jr, Chiu A. T., Duncia J. V., Carini D. J., Wexler R. R., Johnson A. L., Timmermans P. B. Hypotensive action of DuP 753, an angiotensin II antagonist, in spontaneously hypertensive rats. Nonpeptide angiotensin II receptor antagonists: X. Hypertension. 1990 May;15(5):459–468. doi: 10.1161/01.hyp.15.5.459. [DOI] [PubMed] [Google Scholar]
- Yang R. H., Jin H., Wyss J. M., Oparil S. Depressor effect of blocking angiotensin subtype 1 receptors in anterior hypothalamus. Hypertension. 1992 May;19(5):475–481. doi: 10.1161/01.hyp.19.5.475. [DOI] [PubMed] [Google Scholar]



