Abstract
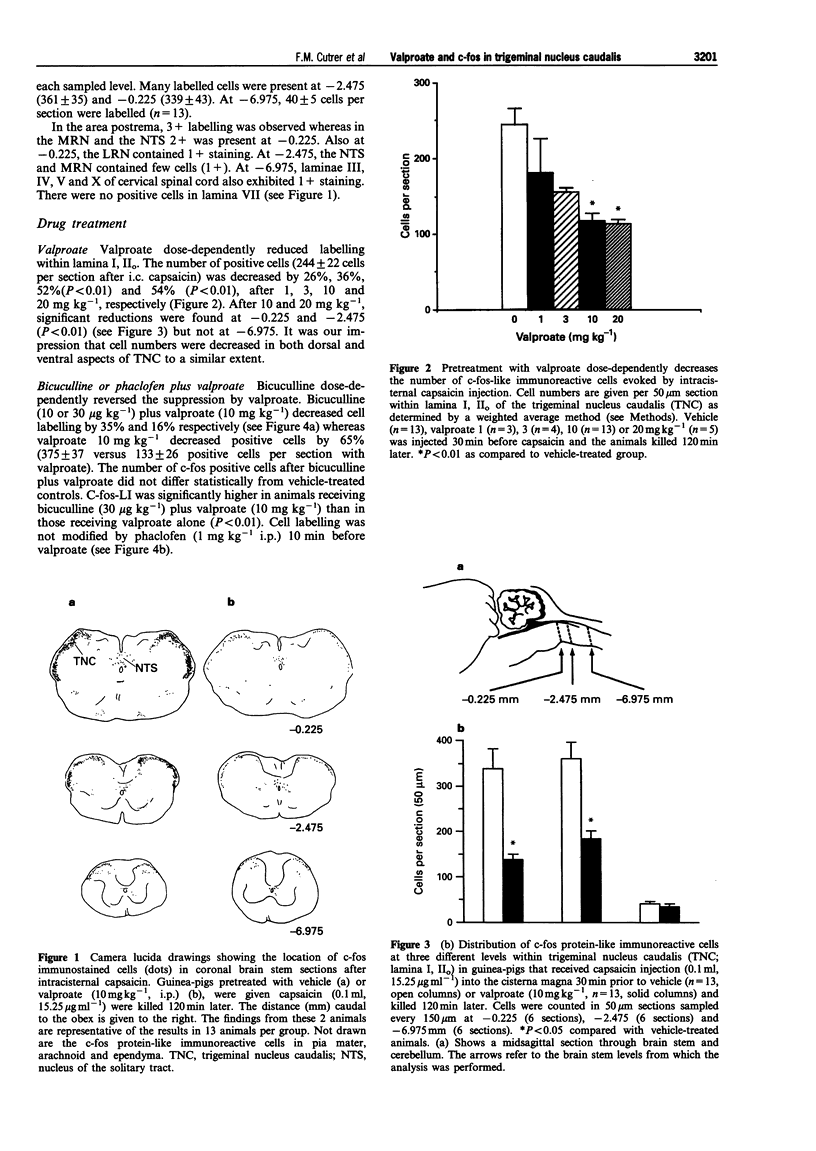
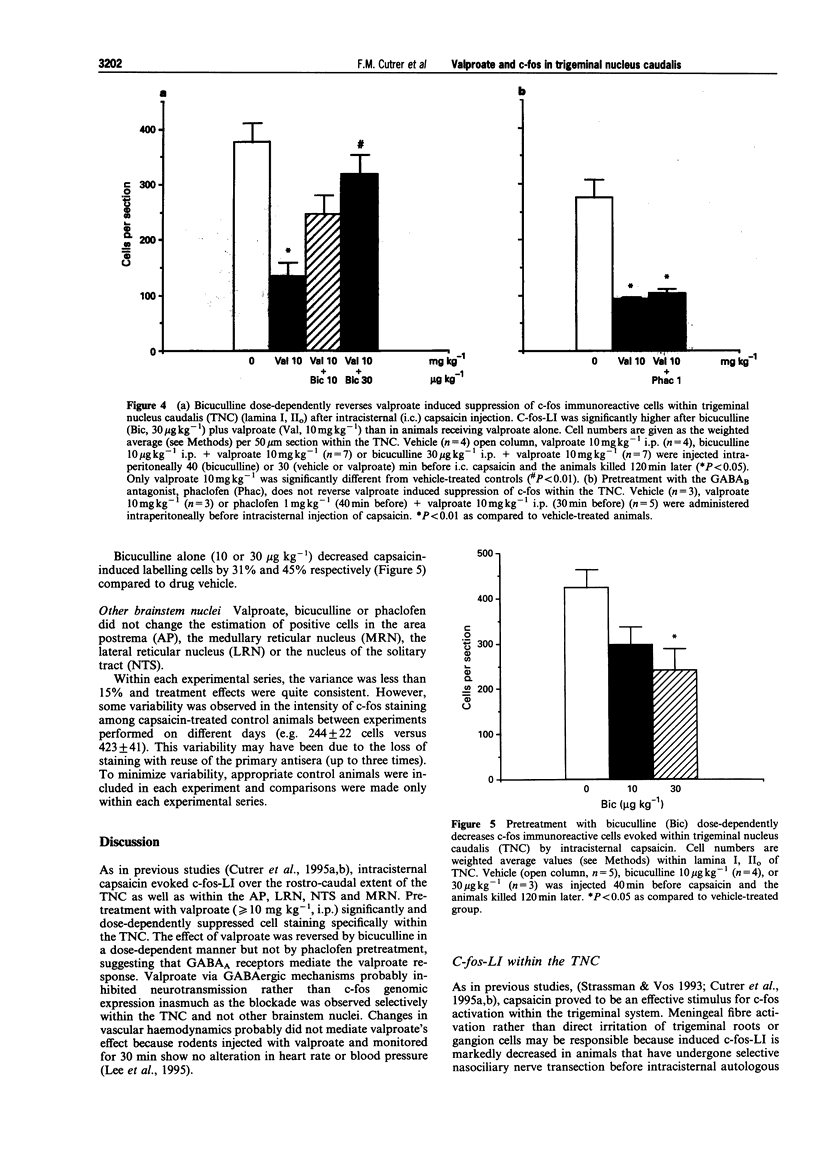
1. Valproic acid, useful in the treatment of migraine, is an inhibitor of gamma aminobutyric acid (GABA) aminotransferase and activator of glutamic acid decarboxylase. Its mechanism in migraine remains obscure. The effects of valproic acid (2-propylpentanoic acid) were examined on the number of cells expressing c-fos-like immunoreactivity (c-fos-LI), a marker of neuronal activation, within the trigeminal nucleus caudalis (lamina I, IIo, TNC) 2 h after intracisternal injection of the irritant, capsaicin (0.1 ml; 15.25 micrograms ml-1), in urethane-anaesthetized Hartley guinea-pigs. Positive cells were counted in eighteen sections (50 microns) at three representative levels (rostral, middle and caudal) within lamina I, IIo of the TNC in 90 animals. 2. Numerous cells were labelled after capsaicin instillation (244 +/- 25; 1 ml; 15.25 mM) but not after capsaicin vehicle (11 +/- 1). Positive cells were also found within the medial reticular nucleus, the area postrema and the nucleus of the solitary tract. A similar distribution has been demonstrated previously after application of intracisternal irritants such as autologous blood or carrageenin. 3. Valproate (> or = 10 mg kg-1, i.p.) reduced labelled cells by 52% (P < 0.05) in lamina I, IIo but not within the area postrema, the nucleus of the solitary tract or the medial reticular nucleus. A similar finding was obtained previously after administration of sumatriptan, dihydroergotamine or the NK1 receptor antagonist RPR 100,893. 4. Pretreatment with bicuculline (30 micrograms kg-1; i.p.), a GABAA antagonist, but not phaclofen (1 mg kg-1) a GABAB antagonist, reversed the effect of valproate and increased c-fos positive cells within lamina I, IIo. Somewhat paradoxically, bicuculline by itself (30 micrograms kg-1 i.p.) decreased the number of labelled cells suggesting that more than a single GABAergic mechanism can suppress c-fos expression. 5. We conclude that the mechanism of action of valproate is mediated via GABAA receptors. Since valproate decreases both c-fos expression and as previously shown, neurogenic inflammation within the meninges, the GABAA receptor complex might provide an important target for drug development in migraine and related headaches.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aran S., Hammond D. L. Antagonism of baclofen-induced antinociception by intrathecal administration of phaclofen or 2-hydroxy-saclofen, but not delta-aminovaleric acid in the rat. J Pharmacol Exp Ther. 1991 Apr;257(1):360–368. [PubMed] [Google Scholar]
- Barber R. P., Vaughn J. E., Roberts E. The cytoarchitecture of GABAergic neurons in rat spinal cord. Brain Res. 1982 Apr 29;238(2):305–328. doi: 10.1016/0006-8993(82)90107-x. [DOI] [PubMed] [Google Scholar]
- Basbaum A. I., Glazer E. J., Oertel W. Immunoreactive glutamic acid decarboxylase in the trigeminal nucleus caudalis of the cat: a light- and electron-microscopic analysis. Somatosens Res. 1986;4(1):77–94. doi: 10.3109/07367228609144599. [DOI] [PubMed] [Google Scholar]
- Bonanno G., Raiteri M. Multiple GABAB receptors. Trends Pharmacol Sci. 1993 Jul;14(7):259–261. doi: 10.1016/0165-6147(93)90124-3. [DOI] [PubMed] [Google Scholar]
- Bowery N. G., Hudson A. L., Price G. W. GABAA and GABAB receptor site distribution in the rat central nervous system. Neuroscience. 1987 Feb;20(2):365–383. doi: 10.1016/0306-4522(87)90098-4. [DOI] [PubMed] [Google Scholar]
- Carlton S. M., Hayes E. S. Light microscopic and ultrastructural analysis of GABA-immunoreactive profiles in the monkey spinal cord. J Comp Neurol. 1990 Oct 8;300(2):162–182. doi: 10.1002/cne.903000203. [DOI] [PubMed] [Google Scholar]
- Cutrer F. M., Moussaoui S., Garret C., Moskowitz M. A. The non-peptide neurokinin-1 antagonist, RPR 100893, decreases c-fos expression in trigeminal nucleus caudalis following noxious chemical meningeal stimulation. Neuroscience. 1995 Feb;64(3):741–750. doi: 10.1016/0306-4522(94)00428-8. [DOI] [PubMed] [Google Scholar]
- Cutrer F. M., Schoenfeld D., Limmroth V., Panahian N., Moskowitz M. A. Suppression by the sumatriptan analogue, CP-122,288 of c-fos immunoreactivity in trigeminal nucleus caudalis induced by intracisternal capsaicin. Br J Pharmacol. 1995 Mar;114(5):987–992. doi: 10.1111/j.1476-5381.1995.tb13302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickenson A. H., Brewer C. M., Hayes N. A. Effects of topical baclofen on C fibre-evoked neuronal activity in the rat dorsal horn. Neuroscience. 1985 Feb;14(2):557–562. doi: 10.1016/0306-4522(85)90310-0. [DOI] [PubMed] [Google Scholar]
- Dragunow M., Robertson H. A. Kindling stimulation induces c-fos protein(s) in granule cells of the rat dentate gyrus. Nature. 1987 Oct 1;329(6138):441–442. doi: 10.1038/329441a0. [DOI] [PubMed] [Google Scholar]
- Godin Y., Heiner L., Mark J., Mandel P. Effects of DI-n-propylacetate, and anticonvulsive compound, on GABA metabolism. J Neurochem. 1969 Jun;16(3):869–873. doi: 10.1111/j.1471-4159.1969.tb08975.x. [DOI] [PubMed] [Google Scholar]
- Hammond D. L., Drower E. J. Effects of intrathecally administered THIP, baclofen and muscimol on nociceptive threshold. Eur J Pharmacol. 1984 Aug 3;103(1-2):121–125. doi: 10.1016/0014-2999(84)90197-3. [DOI] [PubMed] [Google Scholar]
- Hammond D. L., Washington J. D. Antagonism of L-baclofen-induced antinociception by CGP 35348 in the spinal cord of the rat. Eur J Pharmacol. 1993 Apr 6;234(2-3):255–262. doi: 10.1016/0014-2999(93)90961-g. [DOI] [PubMed] [Google Scholar]
- Hering R., Kuritzky A. Sodium valproate in the prophylactic treatment of migraine: a double-blind study versus placebo. Cephalalgia. 1992 Apr;12(2):81–84. doi: 10.1046/j.1468-2982.1992.1202081.x. [DOI] [PubMed] [Google Scholar]
- Hiura A., Ishizuka H., Villalobos E. L. GABAergic neurons in the mouse superficial dorsal horn with special emphasis on their relation to primary afferent central terminals. Arch Histol Cytol. 1991 May;54(2):195–206. doi: 10.1679/aohc.54.195. [DOI] [PubMed] [Google Scholar]
- Hunt S. P., Pini A., Evan G. Induction of c-fos-like protein in spinal cord neurons following sensory stimulation. Nature. 1987 Aug 13;328(6131):632–634. doi: 10.1038/328632a0. [DOI] [PubMed] [Google Scholar]
- Imai H., Okuno T., Wu J. Y., Lee T. J. GABAergic innervation in cerebral blood vessels: an immunohistochemical demonstration of L-glutamic acid decarboxylase and GABA transaminase. J Cereb Blood Flow Metab. 1991 Jan;11(1):129–134. doi: 10.1038/jcbfm.1991.15. [DOI] [PubMed] [Google Scholar]
- Jensen R., Brinck T., Olesen J. Sodium valproate has a prophylactic effect in migraine without aura: a triple-blind, placebo-controlled crossover study. Neurology. 1994 Apr;44(4):647–651. doi: 10.1212/wnl.44.4.647. [DOI] [PubMed] [Google Scholar]
- Lee W. S., Limmroth V., Ayata C., Cutrer F. M., Waeber C., Yu X., Moskowitz M. A. Peripheral GABAA receptor-mediated effects of sodium valproate on dural plasma protein extravasation to substance P and trigeminal stimulation. Br J Pharmacol. 1995 Sep;116(1):1661–1667. doi: 10.1111/j.1476-5381.1995.tb16388.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H., Llewellyn-Smith I. J., Pilowsky P., Basbaum A. I. Ultrastructural evidence for GABA-mediated disinhibitory circuits in the spinal cord of the cat. Neurosci Lett. 1992 Apr 13;138(1):183–187. doi: 10.1016/0304-3940(92)90501-w. [DOI] [PubMed] [Google Scholar]
- Löscher W. Valproate induced changes in GABA metabolism at the subcellular level. Biochem Pharmacol. 1981 Jun 1;30(11):1364–1366. doi: 10.1016/0006-2952(81)90323-3. [DOI] [PubMed] [Google Scholar]
- Macdonald R. L., Olsen R. W. GABAA receptor channels. Annu Rev Neurosci. 1994;17:569–602. doi: 10.1146/annurev.ne.17.030194.003033. [DOI] [PubMed] [Google Scholar]
- Magoul R., Onteniente B., Geffard M., Calas A. Anatomical distribution and ultrastructural organization of the GABAergic system in the rat spinal cord. An immunocytochemical study using anti-GABA antibodies. Neuroscience. 1987 Mar;20(3):1001–1009. doi: 10.1016/0306-4522(87)90258-2. [DOI] [PubMed] [Google Scholar]
- Menétrey D., Gannon A., Levine J. D., Basbaum A. I. Expression of c-fos protein in interneurons and projection neurons of the rat spinal cord in response to noxious somatic, articular, and visceral stimulation. J Comp Neurol. 1989 Jul 8;285(2):177–195. doi: 10.1002/cne.902850203. [DOI] [PubMed] [Google Scholar]
- Morgan J. I., Curran T. Stimulus-transcription coupling in the nervous system: involvement of the inducible proto-oncogenes fos and jun. Annu Rev Neurosci. 1991;14:421–451. doi: 10.1146/annurev.ne.14.030191.002225. [DOI] [PubMed] [Google Scholar]
- Nozaki K., Boccalini P., Moskowitz M. A. Expression of c-fos-like immunoreactivity in brainstem after meningeal irritation by blood in the subarachnoid space. Neuroscience. 1992 Aug;49(3):669–680. doi: 10.1016/0306-4522(92)90235-t. [DOI] [PubMed] [Google Scholar]
- Nozaki K., Moskowitz M. A., Boccalini P. CP-93,129, sumatriptan, dihydroergotamine block c-fos expression within rat trigeminal nucleus caudalis caused by chemical stimulation of the meninges. Br J Pharmacol. 1992 Jun;106(2):409–415. doi: 10.1111/j.1476-5381.1992.tb14348.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacios J. M., Wamsley J. K., Kuhar M. J. High affinity GABA receptors-autoradiographic localization. Brain Res. 1981 Oct 19;222(2):285–307. doi: 10.1016/0006-8993(81)91034-9. [DOI] [PubMed] [Google Scholar]
- Presley R. W., Menétrey D., Levine J. D., Basbaum A. I. Systemic morphine suppresses noxious stimulus-evoked Fos protein-like immunoreactivity in the rat spinal cord. J Neurosci. 1990 Jan;10(1):323–335. doi: 10.1523/JNEUROSCI.10-01-00323.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price G. W., Kelly J. S., Bowery N. G. The location of GABAB receptor binding sites in mammalian spinal cord. Synapse. 1987;1(6):530–538. doi: 10.1002/syn.890010605. [DOI] [PubMed] [Google Scholar]
- Roberts L. A., Beyer C., Komisaruk B. R. Nociceptive responses to altered GABAergic activity at the spinal cord. Life Sci. 1986 Nov 3;39(18):1667–1674. doi: 10.1016/0024-3205(86)90164-5. [DOI] [PubMed] [Google Scholar]
- Sluka K. A., Willis W. D., Westlund K. N. Inflammation-induced release of excitatory amino acids is prevented by spinal administration of a GABAA but not by a GABAB receptor antagonist in rats. J Pharmacol Exp Ther. 1994 Oct;271(1):76–82. [PubMed] [Google Scholar]
- Strassman A. M., Vos B. P. Somatotopic and laminar organization of fos-like immunoreactivity in the medullary and upper cervical dorsal horn induced by noxious facial stimulation in the rat. J Comp Neurol. 1993 May 22;331(4):495–516. doi: 10.1002/cne.903310406. [DOI] [PubMed] [Google Scholar]
- Szabat E., Soinila S., Häppölä O., Linnala A., Virtanen I. A new monoclonal antibody against the GABA-protein conjugate shows immunoreactivity in sensory neurons of the rat. Neuroscience. 1992;47(2):409–420. doi: 10.1016/0306-4522(92)90255-z. [DOI] [PubMed] [Google Scholar]
- Uemura Y., Kowall N. W., Moskowitz M. A. Focal ischemia in rats causes time-dependent expression of c-fos protein immunoreactivity in widespread regions of ipsilateral cortex. Brain Res. 1991 Jun 21;552(1):99–105. doi: 10.1016/0006-8993(91)90665-i. [DOI] [PubMed] [Google Scholar]
- Waldvogel H. J., Faull R. L., Jansen K. L., Dragunow M., Richards J. G., Mohler H., Streit P. GABA, GABA receptors and benzodiazepine receptors in the human spinal cord: an autoradiographic and immunohistochemical study at the light and electron microscopic levels. Neuroscience. 1990;39(2):361–385. doi: 10.1016/0306-4522(90)90274-8. [DOI] [PubMed] [Google Scholar]