Abstract
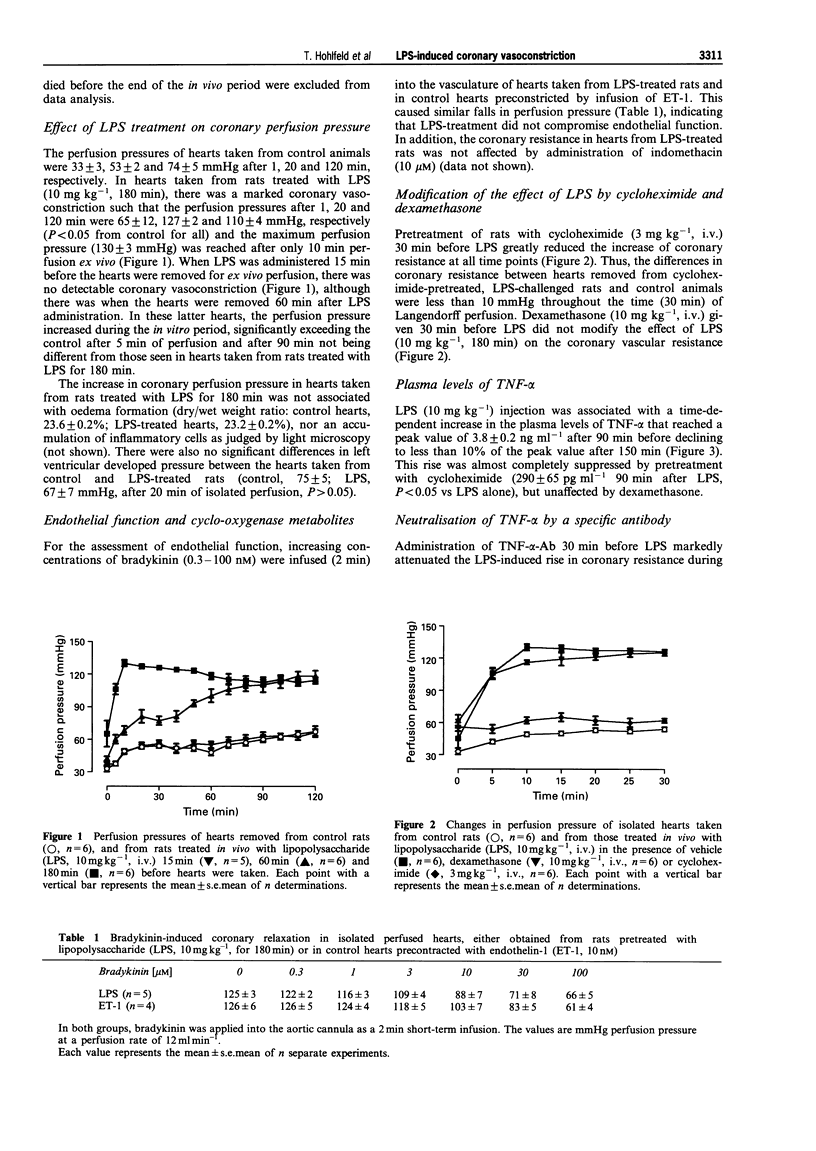
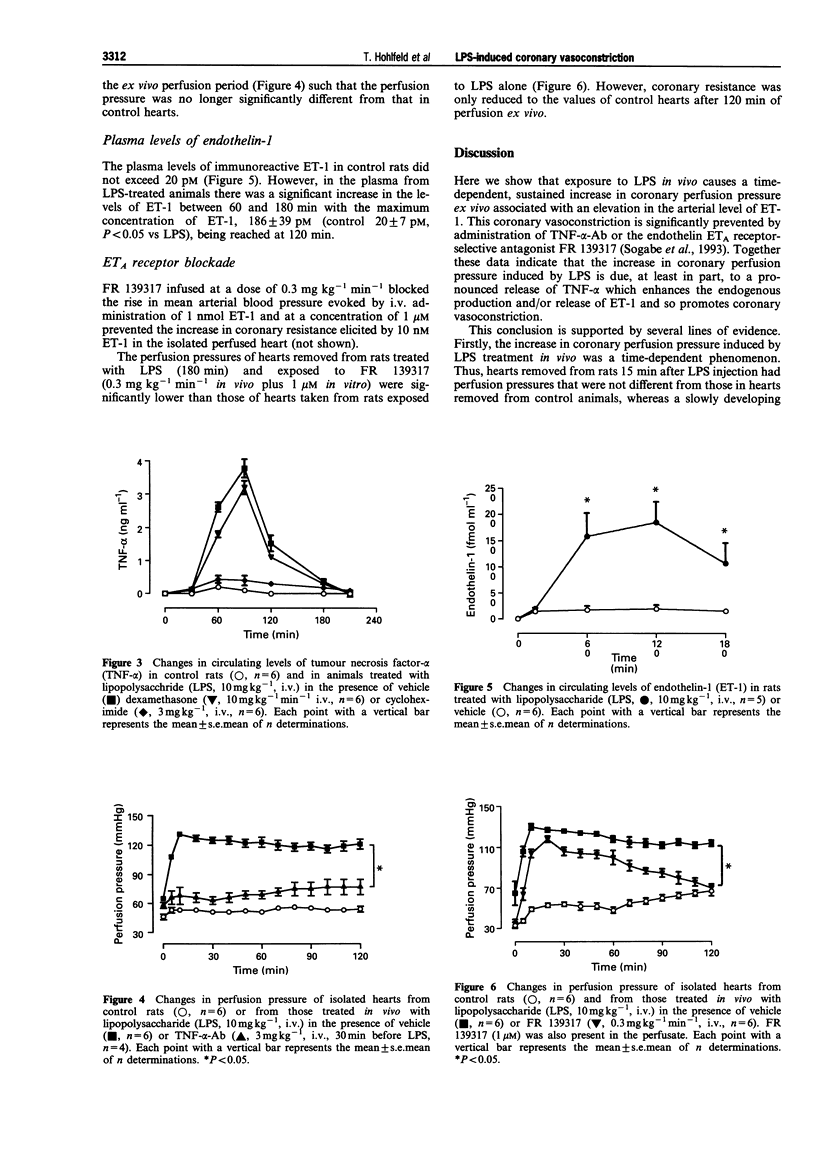
1. Inflammatory disease states predispose to myocardial infarction. Here we have investigated the effects of a systemic inflammatory response syndrome, i.e. lipopolysaccharide (LPS)-induced circulatory shock in rats, on coronary vascular tone. 2. Anaesthetized rats were given LPS (10 mg kg-1, i.v.) and the hearts excised 180 min later for isolated perfusion at constant flow by the Langendorff technique. Once the ex vivo perfusion was started, the perfusion pressure strongly increased in these hearts compared to hearts from control rats (130 +/- 3 vs. 49 +/- 3 mmHg after 10 min). This increase in coronary resistance was not associated with a reduction in endothelial cell function, for the vasodilator responses to bradykinin were unchanged. 3. When hearts were removed 30 min after the injection of LPS, the LPS-induced rise in perfusion pressure was delayed. No changes in perfusion pressure were seen when the hearts were removed 15 min after the injection of LPS. Pre-treatment with cycloheximide or an anti-tumour necrosis factor-alpha (TNF-alpha) antibody or continuous infusion in vivo and in vitro of the endothelin ETA receptor selective antagonist FR 139317, greatly decreased the increase in coronary vascular resistance induced by LPS. 4. These data suggest that TNF-alpha may induce the release of endothelin-1 (ET-1) and that this mediates at least part of the coronary vasoconstriction. This hypothesis is supported by the demonstration that LPS administration increased the circulating levels of both TNF-alpha and ET-1. 5. We conclude, therefore, that in inflammatory disease states, such as LPS-induced septic shock, there is the sequential release of TNF-alpha and endothelin-1 which leads to an increase in coronary vascular tone and so a predisposition to myocardial ischaemia. Inactivation of TNF-alpha by an antibody as well as ETA receptor blockade by a selective antagonist may effectively interfere with this pathway.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benedict C. R., Grahame-Smith D. G. Plasma noradrenaline and adrenaline concentrations and dopamine-beta-hydroxylase activity in patients with shock due to septicaemia, trauma and haemorrhage. Q J Med. 1978 Jan;47(185):1–20. [PubMed] [Google Scholar]
- Beutler B., Krochin N., Milsark I. W., Luedke C., Cerami A. Control of cachectin (tumor necrosis factor) synthesis: mechanisms of endotoxin resistance. Science. 1986 May 23;232(4753):977–980. doi: 10.1126/science.3754653. [DOI] [PubMed] [Google Scholar]
- Fiedler V. B., Loof I., Sander E., Voehringer V., Galanos C., Fournel M. A. Monoclonal antibody to tumor necrosis factor--alpha prevents lethal endotoxin sepsis in adult rhesus monkeys. J Lab Clin Med. 1992 Oct;120(4):574–588. [PubMed] [Google Scholar]
- Foster S. J., McCormick L. M., Ntolosi B. A., Campbell D. Production of TNF alpha by LPS-stimulated murine, rat and human blood and its pharmacological modulation. Agents Actions. 1993;38(Spec No):C77–C79. doi: 10.1007/BF01991143. [DOI] [PubMed] [Google Scholar]
- Han J. J., Windsor A., Drenning D. H., Leeper-Woodford S., Mullen P. G., Bechard D. E., Sugerman H. J., Fowler A. A., 3rd Release of endothelin in relation to tumor necrosis factor-alpha in porcine Pseudomonas aeruginosa-induced septic shock. Shock. 1994 May;1(5):343–346. doi: 10.1097/00024382-199405000-00004. [DOI] [PubMed] [Google Scholar]
- Hansen P. R., Svendsen J. H., Høyer S., Kharazmi A., Bendtzen K., Haunsø S. Tumor necrosis factor-alpha increases myocardial microvascular transport in vivo. Am J Physiol. 1994 Jan;266(1 Pt 2):H60–H67. doi: 10.1152/ajpheart.1994.266.1.H60. [DOI] [PubMed] [Google Scholar]
- Hardie E. M., Olson N. C. Prostaglandin and thromboxane levels during endotoxin-induced respiratory failure in pigs. Prostaglandins Leukot Med. 1987 Aug;28(3):255–265. doi: 10.1016/0262-1746(87)90115-6. [DOI] [PubMed] [Google Scholar]
- Hofsli E., Lamvik J., Nissen-Meyer J. Evidence that tumour necrosis factor (TNF) is not constitutively present in vivo. The association of TNF with freshly isolated monocytes reflects a rapid in vitro production. Scand J Immunol. 1988 Oct;28(4):435–441. doi: 10.1111/j.1365-3083.1988.tb01473.x. [DOI] [PubMed] [Google Scholar]
- Hori S., Komatsu Y., Shigemoto R., Mizuno N., Nakanishi S. Distinct tissue distribution and cellular localization of two messenger ribonucleic acids encoding different subtypes of rat endothelin receptors. Endocrinology. 1992 Apr;130(4):1885–1895. doi: 10.1210/endo.130.4.1312429. [DOI] [PubMed] [Google Scholar]
- Julou-Schaeffer G., Gray G. A., Fleming I., Schott C., Parratt J. R., Stoclet J. C. Loss of vascular responsiveness induced by endotoxin involves L-arginine pathway. Am J Physiol. 1990 Oct;259(4 Pt 2):H1038–H1043. doi: 10.1152/ajpheart.1990.259.4.H1038. [DOI] [PubMed] [Google Scholar]
- Kilbourn R. G., Gross S. S., Jubran A., Adams J., Griffith O. W., Levi R., Lodato R. F. NG-methyl-L-arginine inhibits tumor necrosis factor-induced hypotension: implications for the involvement of nitric oxide. Proc Natl Acad Sci U S A. 1990 May;87(9):3629–3632. doi: 10.1073/pnas.87.9.3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilbourn R. G., Jubran A., Gross S. S., Griffith O. W., Levi R., Adams J., Lodato R. F. Reversal of endotoxin-mediated shock by NG-methyl-L-arginine, an inhibitor of nitric oxide synthesis. Biochem Biophys Res Commun. 1990 Nov 15;172(3):1132–1138. doi: 10.1016/0006-291x(90)91565-a. [DOI] [PubMed] [Google Scholar]
- Klemm P., Warner T. D., Hohlfeld T., Corder R., Vane J. R. Endothelin 1 mediates ex vivo coronary vasoconstriction caused by exogenous and endogenous cytokines. Proc Natl Acad Sci U S A. 1995 Mar 28;92(7):2691–2695. doi: 10.1073/pnas.92.7.2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemm P., Warner T. D., Willis D., Moore A. R., Vane J. R. Coronary vasoconstriction in vitro in the hearts of polyarthritic rats: effectiveness of in vivo treatment with the endothelin receptor antagonist SB 209670. Br J Pharmacol. 1995 Apr;114(7):1327–1328. doi: 10.1111/j.1476-5381.1995.tb13351.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kragel A. H., Travis W. D., Steis R. G., Rosenberg S. A., Roberts W. C. Myocarditis or acute myocardial infarction associated with interleukin-2 therapy for cancer. Cancer. 1990 Oct 1;66(7):1513–1516. doi: 10.1002/1097-0142(19901001)66:7<1513::aid-cncr2820660713>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Leinonen M., Linnanmäki E., Mattila K., Nieminen M. S., Valtonen V., Leirisalo-Repo M., Saikku P. Circulating immune complexes containing chlamydial lipopolysaccharide in acute myocardial infarction. Microb Pathog. 1990 Jul;9(1):67–73. doi: 10.1016/0882-4010(90)90042-o. [DOI] [PubMed] [Google Scholar]
- Leung D. Y. The potential role of cytokine-mediated vascular endothelial activation in the pathogenesis of Kawasaki disease. Acta Paediatr Jpn. 1991 Dec;33(6):739–744. doi: 10.1111/j.1442-200x.1991.tb02602.x. [DOI] [PubMed] [Google Scholar]
- Lin C. Y., Lin C. C., Hwang B., Chiang B. N. The changes of interleukin-2, tumour necrotic factor and gamma-interferon production among patients with Kawasaki disease. Eur J Pediatr. 1991 Jan;150(3):179–182. doi: 10.1007/BF01963561. [DOI] [PubMed] [Google Scholar]
- Lundberg J. M., Ahlborg G., Hemsén A., Nisell H., Lunell N. O., Pernow J., Rudehill A., Weitzberg E. Evidence for release of endothelin-1 in pigs and humans. J Cardiovasc Pharmacol. 1991;17 (Suppl 7):S350–S353. doi: 10.1097/00005344-199100177-00100. [DOI] [PubMed] [Google Scholar]
- Marsden P. A., Brenner B. M. Transcriptional regulation of the endothelin-1 gene by TNF-alpha. Am J Physiol. 1992 Apr;262(4 Pt 1):C854–C861. doi: 10.1152/ajpcell.1992.262.4.C854. [DOI] [PubMed] [Google Scholar]
- McDonough K. H., Brumfield B. A., Lang C. H. In vitro myocardial performance after lethal and nonlethal doses of endotoxin. Am J Physiol. 1986 Feb;250(2 Pt 2):H240–H246. doi: 10.1152/ajpheart.1986.250.2.H240. [DOI] [PubMed] [Google Scholar]
- Morise Z., Ueda M., Aiura K., Endo M., Kitajima M. Pathophysiologic role of endothelin-1 in renal function in rats with endotoxin shock. Surgery. 1994 Feb;115(2):199–204. [PubMed] [Google Scholar]
- Myhre U., Pettersen J. T., Risøe C., Giercksky K. E. Endothelin-1 and endotoxemia. J Cardiovasc Pharmacol. 1993;22 (Suppl 8):S291–S294. doi: 10.1097/00005344-199322008-00076. [DOI] [PubMed] [Google Scholar]
- Nakamura T., Kasai K., Sekiguchi Y., Banba N., Takahashi K., Emoto T., Hattori Y., Shimoda S. Elevation of plasma endothelin concentrations during endotoxin shock in dogs. Eur J Pharmacol. 1991 Dec 3;205(3):277–282. doi: 10.1016/0014-2999(91)90910-i. [DOI] [PubMed] [Google Scholar]
- Natanson C., Hoffman W. D., Suffredini A. F., Eichacker P. Q., Danner R. L. Selected treatment strategies for septic shock based on proposed mechanisms of pathogenesis. Ann Intern Med. 1994 May 1;120(9):771–783. doi: 10.7326/0003-4819-120-9-199405010-00009. [DOI] [PubMed] [Google Scholar]
- Nieminen M. S., Mattila K., Valtonen V. Infection and inflammation as risk factors for myocardial infarction. Eur Heart J. 1993 Dec;14 (Suppl K):12–16. [PubMed] [Google Scholar]
- Nora R., Abrams J. S., Tait N. S., Hiponia D. J., Silverman H. J. Myocardial toxic effects during recombinant interleukin-2 therapy. J Natl Cancer Inst. 1989 Jan 4;81(1):59–63. doi: 10.1093/jnci/81.1.59. [DOI] [PubMed] [Google Scholar]
- Oettinger W., Berger D., Beger H. G. The clinical significance of prostaglandins and thromboxane as mediators of septic shock. Klin Wochenschr. 1987 Jan 15;65(2):61–68. doi: 10.1007/BF01745474. [DOI] [PubMed] [Google Scholar]
- Pernow J., Hemsén A., Hallén A., Lundberg J. M. Release of endothelin-like immunoreactivity in relation to neuropeptide Y and catecholamines during endotoxin shock and asphyxia in the pig. Acta Physiol Scand. 1990 Nov;140(3):311–322. doi: 10.1111/j.1748-1716.1990.tb09005.x. [DOI] [PubMed] [Google Scholar]
- Pollock D. M., Divish B. J., Opgenorth T. J. Stimulation of endogenous endothelin release in the anesthetized rat. J Cardiovasc Pharmacol. 1993;22 (Suppl 8):S295–S298. doi: 10.1097/00005344-199322008-00077. [DOI] [PubMed] [Google Scholar]
- Rees D. D., Cellek S., Palmer R. M., Moncada S. Dexamethasone prevents the induction by endotoxin of a nitric oxide synthase and the associated effects on vascular tone: an insight into endotoxin shock. Biochem Biophys Res Commun. 1990 Dec 14;173(2):541–547. doi: 10.1016/s0006-291x(05)80068-3. [DOI] [PubMed] [Google Scholar]
- Saikku P. Chlamydia pneumoniae infection as a risk factor in acute myocardial infarction. Eur Heart J. 1993 Dec;14 (Suppl K):62–65. [PubMed] [Google Scholar]
- Schini V. B., Hendrickson H., Heublein D. M., Burnett J. C., Jr, Vanhoutte P. M. Thrombin enhances the release of endothelin from cultured porcine aortic endothelial cells. Eur J Pharmacol. 1989 Jun 20;165(2-3):333–334. doi: 10.1016/0014-2999(89)90733-4. [DOI] [PubMed] [Google Scholar]
- Sheagren J. N. Corticosteroids for the treatment of septic shock. Infect Dis Clin North Am. 1991 Dec;5(4):875–882. [PubMed] [Google Scholar]
- Silva A. T., Bayston K. F., Cohen J. Prophylactic and therapeutic effects of a monoclonal antibody to tumor necrosis factor-alpha in experimental gram-negative shock. J Infect Dis. 1990 Aug;162(2):421–427. doi: 10.1093/infdis/162.2.421. [DOI] [PubMed] [Google Scholar]
- Sogabe K., Nirei H., Shoubo M., Nomoto A., Ao S., Notsu Y., Ono T. Pharmacological profile of FR139317, a novel, potent endothelin ETA receptor antagonist. J Pharmacol Exp Ther. 1993 Mar;264(3):1040–1046. [PubMed] [Google Scholar]
- Sugiura M., Inagami T., Kon V. Endotoxin stimulates endothelin-release in vivo and in vitro as determined by radioimmunoassay. Biochem Biophys Res Commun. 1989 Jun 30;161(3):1220–1227. doi: 10.1016/0006-291x(89)91372-7. [DOI] [PubMed] [Google Scholar]
- Szabó C., Mitchell J. A., Thiemermann C., Vane J. R. Nitric oxide-mediated hyporeactivity to noradrenaline precedes the induction of nitric oxide synthase in endotoxin shock. Br J Pharmacol. 1993 Mar;108(3):786–792. doi: 10.1111/j.1476-5381.1993.tb12879.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiemermann C., Vane J. Inhibition of nitric oxide synthesis reduces the hypotension induced by bacterial lipopolysaccharides in the rat in vivo. Eur J Pharmacol. 1990 Jul 17;182(3):591–595. doi: 10.1016/0014-2999(90)90062-b. [DOI] [PubMed] [Google Scholar]
- Tomita S., Myones B. L., Shulman S. T. In vitro correlates of the L. casei animal model of Kawasaki disease. J Rheumatol. 1993 Feb;20(2):362–367. [PubMed] [Google Scholar]
- Vemulapalli S., Chiu P. J., Rivelli M., Foster C. J., Sybertz E. J. Modulation of circulating endothelin levels in hypertension and endotoxemia in rats. J Cardiovasc Pharmacol. 1991 Dec;18(6):895–903. doi: 10.1097/00005344-199112000-00017. [DOI] [PubMed] [Google Scholar]
- Vincent J. L., Bakker J., Marécaux G., Schandene L., Kahn R. J., Dupont E. Administration of anti-TNF antibody improves left ventricular function in septic shock patients. Results of a pilot study. Chest. 1992 Mar;101(3):810–815. doi: 10.1378/chest.101.3.810. [DOI] [PubMed] [Google Scholar]
- Voerman H. J., Stehouwer C. D., van Kamp G. J., Strack van Schijndel R. J., Groeneveld A. B., Thijs L. G. Plasma endothelin levels are increased during septic shock. Crit Care Med. 1992 Aug;20(8):1097–1101. doi: 10.1097/00003246-199208000-00005. [DOI] [PubMed] [Google Scholar]
- Windsor A. C., Mullen P. G., Walsh C. J., Fisher B. J., Blocher C. R., Jesmok G., Fowler A. A., 3rd, Sugerman H. J. Delayed tumor necrosis factor alpha blockade attenuates pulmonary dysfunction and metabolic acidosis associated with experimental gram-negative sepsis. Arch Surg. 1994 Jan;129(1):80–89. doi: 10.1001/archsurg.1994.01420250092012. [DOI] [PubMed] [Google Scholar]


