Abstract
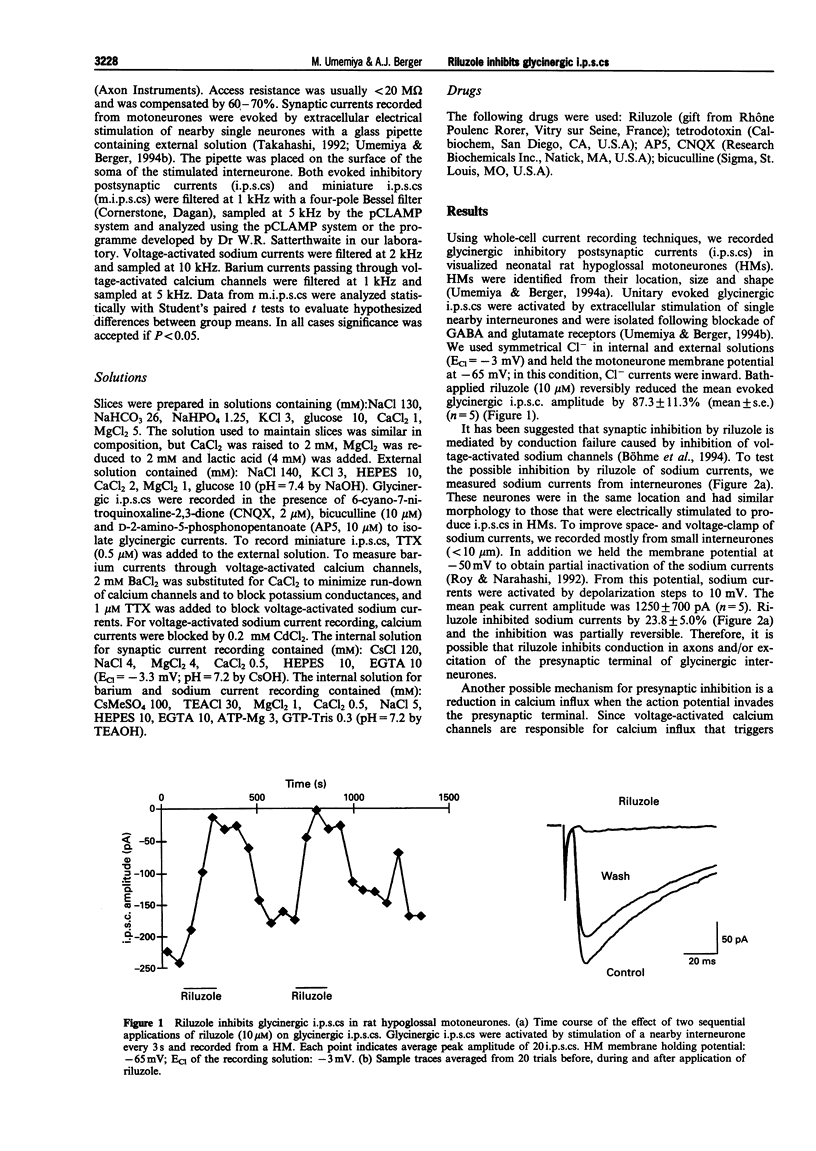
1. Riluzole has been shown to have beneficial effects in motoneurone disease, yet its effect on motoneurones is not known. To address this question, we investigated synaptic modulation by riluzole in hypoglossal motoneurones by recording glycinergic inhibitory postsynaptic currents evoked by stimulation of nearby single interneurones. 2. Glycinergic inhibitory postsynaptic currents were evoked by electrical stimulation of single interneurones and were recorded from visually identified hypoglossal motoneurones. Riluzole (10 microM) inhibited mean amplitude of evoked glycinergic inhibitory postsynaptic currents by 87%. 3. We found that riluzole suppressed sodium currents in brainstem interneurones by 23.8%. Riluzole did not modulate barium currents through voltage-activated calcium channels (98% of control). Therefore, the effect of riluzole on synaptic transmission may be mediated, in part, by stabilizing presynaptic neurones through inhibition of voltage-activated sodium currents. 4. In the presence of tetrodotoxin (0.5 microM), riluzole reduced the frequency (1.2 Hz in control to 0.6 Hz in riluzole) of spontaneous transmitter release recorded in motoneurones. 5. Riluzole was found to have no effect on mean miniature inhibitory postsynaptic current amplitude, therefore the reduction in spontaneous transmitter release cannot be due to an action on postsynaptic glycine receptors. 6. We conclude that riluzole inhibits synaptic transmission presynaptically, independent of a reduction in the excitation of presynaptic neurones.
Full text
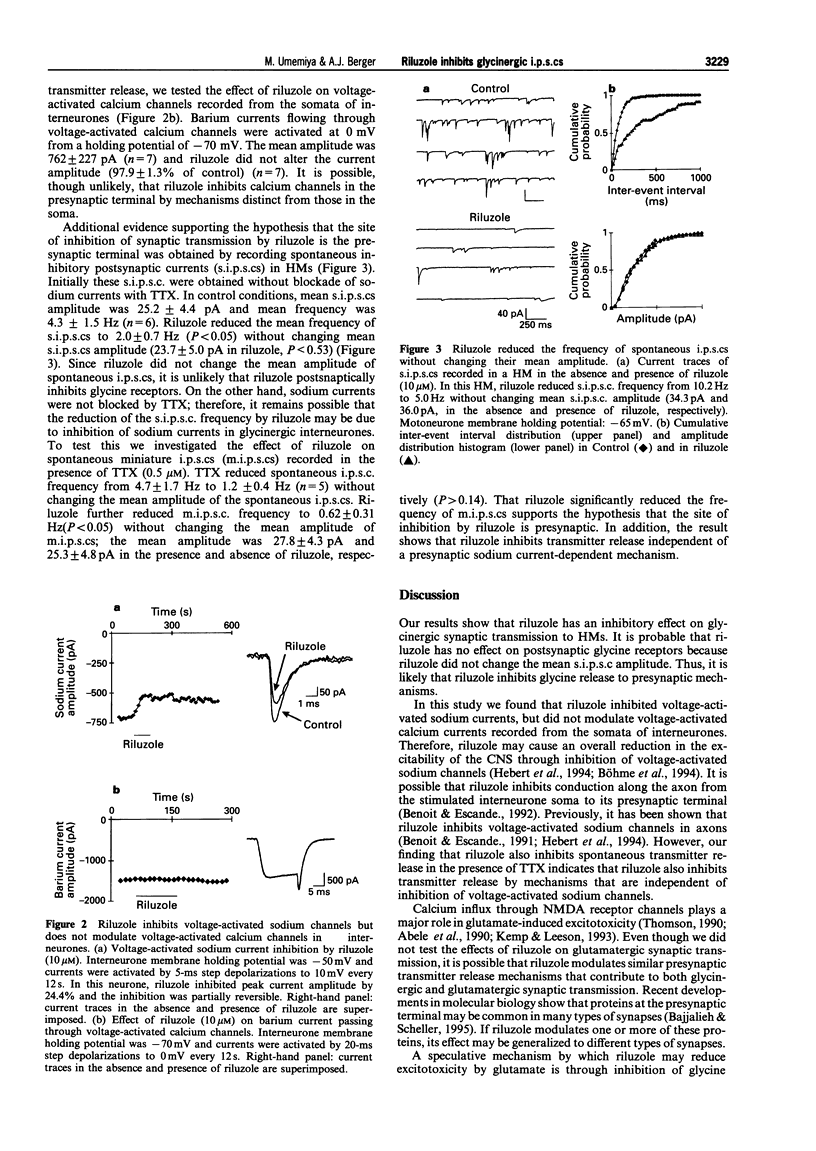
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abele A. E., Scholz K. P., Scholz W. K., Miller R. J. Excitotoxicity induced by enhanced excitatory neurotransmission in cultured hippocampal pyramidal neurons. Neuron. 1990 Mar;4(3):413–419. doi: 10.1016/0896-6273(90)90053-i. [DOI] [PubMed] [Google Scholar]
- Bajjalieh S. M., Scheller R. H. The biochemistry of neurotransmitter secretion. J Biol Chem. 1995 Feb 3;270(5):1971–1974. doi: 10.1074/jbc.270.5.1971. [DOI] [PubMed] [Google Scholar]
- Barbour B., Keller B. U., Llano I., Marty A. Prolonged presence of glutamate during excitatory synaptic transmission to cerebellar Purkinje cells. Neuron. 1994 Jun;12(6):1331–1343. doi: 10.1016/0896-6273(94)90448-0. [DOI] [PubMed] [Google Scholar]
- Benoit E., Escande D. Riluzole specifically blocks inactivated Na channels in myelinated nerve fibre. Pflugers Arch. 1991 Dec;419(6):603–609. doi: 10.1007/BF00370302. [DOI] [PubMed] [Google Scholar]
- Bensimon G., Lacomblez L., Meininger V. A controlled trial of riluzole in amyotrophic lateral sclerosis. ALS/Riluzole Study Group. N Engl J Med. 1994 Mar 3;330(9):585–591. doi: 10.1056/NEJM199403033300901. [DOI] [PubMed] [Google Scholar]
- Chéramy A., Barbeito L., Godeheu G., Glowinski J. Riluzole inhibits the release of glutamate in the caudate nucleus of the cat in vivo. Neurosci Lett. 1992 Dec 7;147(2):209–212. doi: 10.1016/0304-3940(92)90597-z. [DOI] [PubMed] [Google Scholar]
- Debono M. W., Le Guern J., Canton T., Doble A., Pradier L. Inhibition by riluzole of electrophysiological responses mediated by rat kainate and NMDA receptors expressed in Xenopus oocytes. Eur J Pharmacol. 1993 Apr 28;235(2-3):283–289. doi: 10.1016/0014-2999(93)90147-a. [DOI] [PubMed] [Google Scholar]
- Doble A., Hubert J. P., Blanchard J. C. Pertussis toxin pretreatment abolishes the inhibitory effect of riluzole and carbachol on D-[3H]aspartate release from cultured cerebellar granule cells. Neurosci Lett. 1992 Jun 22;140(2):251–254. doi: 10.1016/0304-3940(92)90114-m. [DOI] [PubMed] [Google Scholar]
- Edwards F. A., Konnerth A., Sakmann B., Takahashi T. A thin slice preparation for patch clamp recordings from neurones of the mammalian central nervous system. Pflugers Arch. 1989 Sep;414(5):600–612. doi: 10.1007/BF00580998. [DOI] [PubMed] [Google Scholar]
- HAGGAR R. A., BARR M. L. Quantitative data on the size of synaptic end-bulbs in the cat's spinal cord. J Comp Neurol. 1950 Aug;93(1):17–35. doi: 10.1002/cne.900930103. [DOI] [PubMed] [Google Scholar]
- Hebert T., Drapeau P., Pradier L., Dunn R. J. Block of the rat brain IIA sodium channel alpha subunit by the neuroprotective drug riluzole. Mol Pharmacol. 1994 May;45(5):1055–1060. [PubMed] [Google Scholar]
- Johnson J. W., Ascher P. Equilibrium and kinetic study of glycine action on the N-methyl-D-aspartate receptor in cultured mouse brain neurons. J Physiol. 1992 Sep;455:339–365. doi: 10.1113/jphysiol.1992.sp019305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp J. A., Leeson P. D. The glycine site of the NMDA receptor--five years on. Trends Pharmacol Sci. 1993 Jan;14(1):20–25. doi: 10.1016/0165-6147(93)90108-v. [DOI] [PubMed] [Google Scholar]
- Martin D., Thompson M. A., Nadler J. V. The neuroprotective agent riluzole inhibits release of glutamate and aspartate from slices of hippocampal area CA1. Eur J Pharmacol. 1993 Dec 21;250(3):473–476. doi: 10.1016/0014-2999(93)90037-i. [DOI] [PubMed] [Google Scholar]
- McBain C. J., Mayer M. L. N-methyl-D-aspartic acid receptor structure and function. Physiol Rev. 1994 Jul;74(3):723–760. doi: 10.1152/physrev.1994.74.3.723. [DOI] [PubMed] [Google Scholar]
- Rowland L. P. Amyotrophic lateral sclerosis. Curr Opin Neurol. 1994 Aug;7(4):310–315. doi: 10.1097/00019052-199408000-00006. [DOI] [PubMed] [Google Scholar]
- Roy M. L., Narahashi T. Differential properties of tetrodotoxin-sensitive and tetrodotoxin-resistant sodium channels in rat dorsal root ganglion neurons. J Neurosci. 1992 Jun;12(6):2104–2111. doi: 10.1523/JNEUROSCI.12-06-02104.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stutzmann J. M., Böhme G. A., Gandolfo G., Gottesmann C., Lafforgue J., Blanchard J. C., Laduron P. M., Lazdunski M. Riluzole prevents hyperexcitability produced by the mast cell degranulating peptide and dendrotoxin I in the rat. Eur J Pharmacol. 1991 Feb 7;193(2):223–229. doi: 10.1016/0014-2999(91)90040-w. [DOI] [PubMed] [Google Scholar]
- Takahashi T. The minimal inhibitory synaptic currents evoked in neonatal rat motoneurones. J Physiol. 1992 May;450:593–611. doi: 10.1113/jphysiol.1992.sp019145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang C. M., Margulis M., Shi Q. Y., Fielding A. Saturation of postsynaptic glutamate receptors after quantal release of transmitter. Neuron. 1994 Dec;13(6):1385–1393. doi: 10.1016/0896-6273(94)90423-5. [DOI] [PubMed] [Google Scholar]
- Thomson A. M. Glycine is a coagonist at the NMDA receptor/channel complex. Prog Neurobiol. 1990;35(1):53–74. doi: 10.1016/0301-0082(90)90040-n. [DOI] [PubMed] [Google Scholar]
- Umemiya M., Berger A. J. Activation of adenosine A1 and A2 receptors differentially modulates calcium channels and glycinergic synaptic transmission in rat brainstem. Neuron. 1994 Dec;13(6):1439–1446. doi: 10.1016/0896-6273(94)90429-4. [DOI] [PubMed] [Google Scholar]
- Umemiya M., Berger A. J. Properties and function of low- and high-voltage-activated Ca2+ channels in hypoglossal motoneurons. J Neurosci. 1994 Sep;14(9):5652–5660. doi: 10.1523/JNEUROSCI.14-09-05652.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyklický L., Jr, Benveniste M., Mayer M. L. Modulation of N-methyl-D-aspartic acid receptor desensitization by glycine in mouse cultured hippocampal neurones. J Physiol. 1990 Sep;428:313–331. doi: 10.1113/jphysiol.1990.sp018214. [DOI] [PMC free article] [PubMed] [Google Scholar]


