Abstract
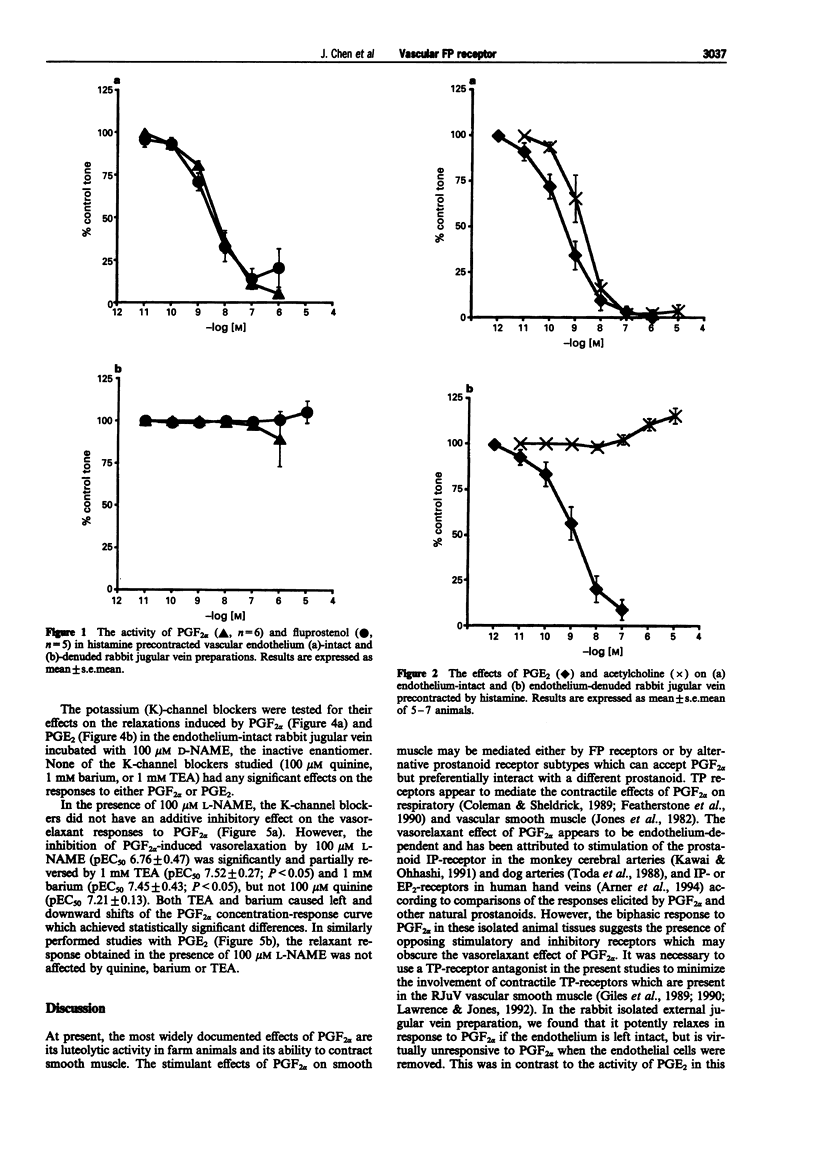
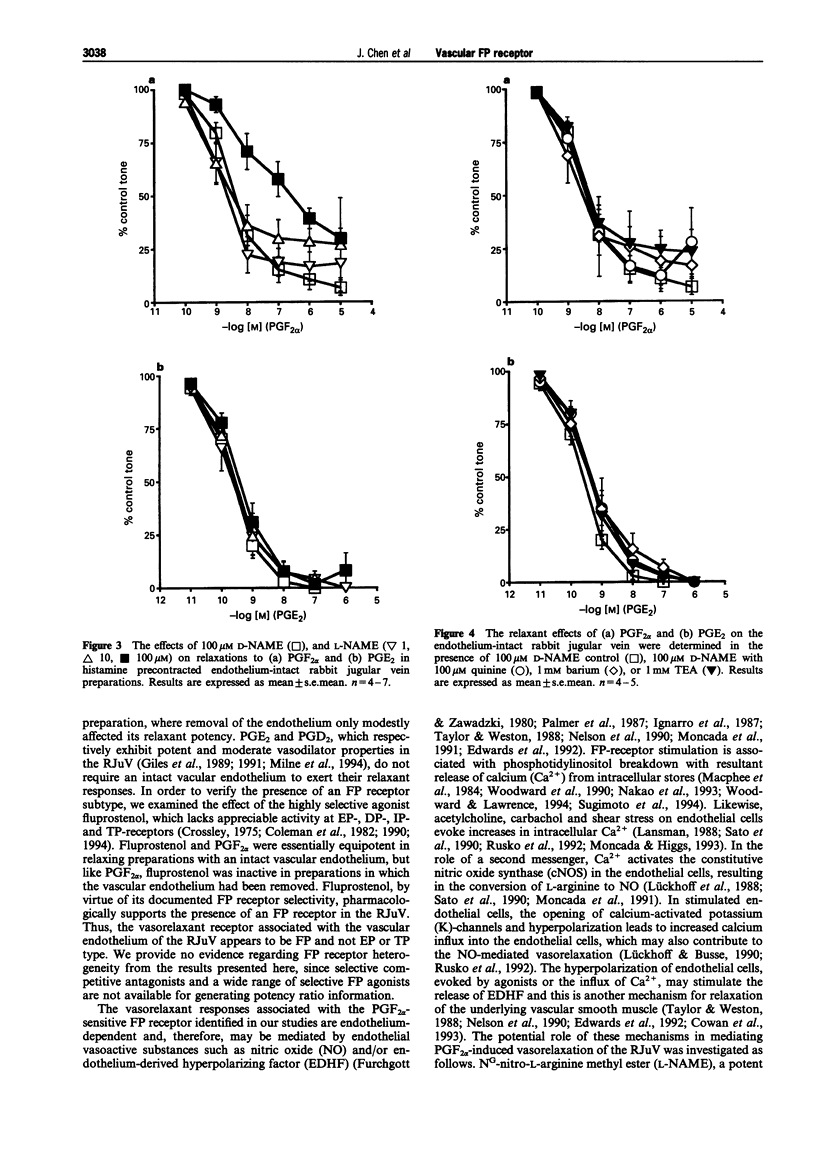
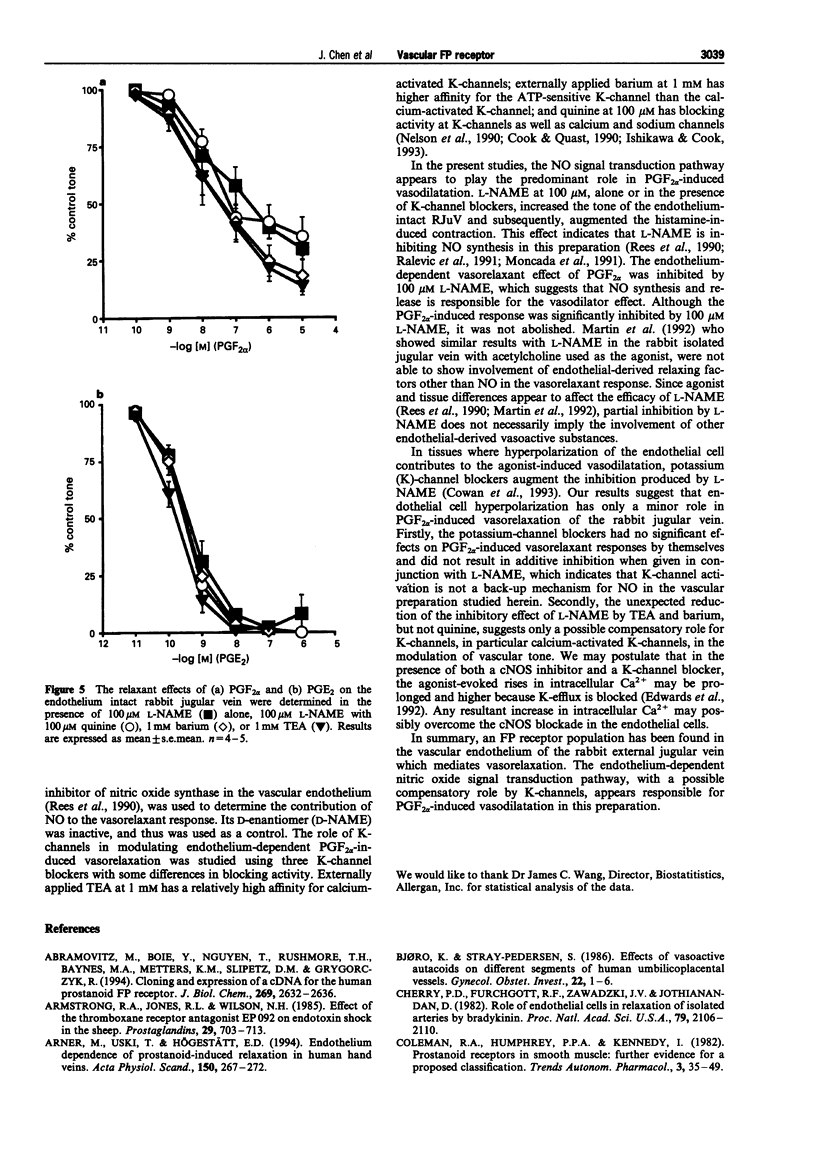
1. Prostaglandin F2 alpha (PGF2 alpha) and its synthetic analogue, fluprostenol, potently relaxed the precontracted isolated jugular vein of the rabbit (RJuV). The vasorelaxant activity of PGF2 alpha and fluprostenol was dependent upon an intact vascular endothelium. Although removal of the vascular endothelium abolished activity associated with PGF2 alpha-like agonists, it did not significantly alter the relaxant effects of prostaglandin E2 (PGE2). 2. The nitric oxide synthase inhibitor, NG-nitro-L-arginine methyl ester (L-NAME), at 100 microM significantly inhibited the endothelium-dependent relaxations induced by PGF2 alpha. Lower doses (1 microM, 10 microM) of L-NAME had little or no effect. The relaxant effects of PGE2 were not affected by L-NAME (1-100 microM). D-NAME at 100 microM was without effect on the vasorelaxant responses to either PGF2 alpha or PGE2. 3. The potassium (K)-channel blockers tetraethylammonium (TEA, 1 mM), barium (1 mM) and quinine (100 microM), each tested in the presence of the inactive enantiomer D-NAME (100 microM) did not significantly affect the response to PGF2 alpha. Unexpectedly, both TEA and barium significantly and partially reversed the inhibitory effects of 100 microM L-NAME, whereas quinine had no effect. In similar studies, none of the three potassium channel blockers had any effect on relaxations elicited by PGE2 when given with D-NAME or L-NAME. 4. These results indicate that the PGF2 alpha-sensitive prostanoid receptors found in the vascular endothelium of the rabbit jugular vein are of the FP-receptor subtype. Nitric oxide (NO) appears to be the predominant messenger involved in PGF2 alpha-induced relaxation of the rabbit jugular vein. Potassium channels may have a minor role in mediating the vasorelaxation response to PGF2 alpha. When both NO synthesis and K-channels are simultaneously blocked, inhibition of PGF2 alpha-induced vasorelaxation by L-NAME is opposed by K-channel blockers. This diminution of the inhibitory effect of L-NAME by TEA and barium suggests that K-channels may possibly serve a compensatory role via the NO pathway.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abramovitz M., Boie Y., Nguyen T., Rushmore T. H., Bayne M. A., Metters K. M., Slipetz D. M., Grygorczyk R. Cloning and expression of a cDNA for the human prostanoid FP receptor. J Biol Chem. 1994 Jan 28;269(4):2632–2636. [PubMed] [Google Scholar]
- Armstrong R. A., Jones R. L., Wilson N. H. Effect of the thromboxane receptor antagonist EP 092 on endotoxin shock in the sheep. Prostaglandins. 1985 May;29(5):703–713. doi: 10.1016/0090-6980(85)90131-5. [DOI] [PubMed] [Google Scholar]
- Arner M., Uski T., Högestätt E. D. Endothelium dependence of prostanoid-induced relaxation in human hand veins. Acta Physiol Scand. 1994 Mar;150(3):267–272. doi: 10.1111/j.1748-1716.1994.tb09686.x. [DOI] [PubMed] [Google Scholar]
- Bjøro K., Stray-Pedersen S. Effects of vasoactive autacoids on different segments of human umbilicoplacental vessels. Gynecol Obstet Invest. 1986;22(1):1–6. doi: 10.1159/000298881. [DOI] [PubMed] [Google Scholar]
- Buzzi M. G., Moskowitz M. A. The antimigraine drug, sumatriptan (GR43175), selectively blocks neurogenic plasma extravasation from blood vessels in dura mater. Br J Pharmacol. 1990 Jan;99(1):202–206. doi: 10.1111/j.1476-5381.1990.tb14679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherry P. D., Furchgott R. F., Zawadzki J. V., Jothianandan D. Role of endothelial cells in relaxation of isolated arteries by bradykinin. Proc Natl Acad Sci U S A. 1982 Mar;79(6):2106–2110. doi: 10.1073/pnas.79.6.2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman R. A., Sheldrick R. L. Prostanoid-induced contraction of human bronchial smooth muscle is mediated by TP-receptors. Br J Pharmacol. 1989 Mar;96(3):688–692. doi: 10.1111/j.1476-5381.1989.tb11869.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman R. A., Smith W. L., Narumiya S. International Union of Pharmacology classification of prostanoid receptors: properties, distribution, and structure of the receptors and their subtypes. Pharmacol Rev. 1994 Jun;46(2):205–229. [PubMed] [Google Scholar]
- Cowan C. L., Palacino J. J., Najibi S., Cohen R. A. Potassium channel-mediated relaxation to acetylcholine in rabbit arteries. J Pharmacol Exp Ther. 1993 Sep;266(3):1482–1489. [PubMed] [Google Scholar]
- Crossley N. S. The synthesis and biological activity of potent, selective luteolytic prostaglandins. Prostaglandins. 1975 Jul;10(1):5–18. doi: 10.1016/0090-6980(75)90090-8. [DOI] [PubMed] [Google Scholar]
- Eglen R. M., Whiting R. L. The action of prostanoid receptor agonists and antagonists on smooth muscle and platelets. Br J Pharmacol. 1988 Jun;94(2):591–601. doi: 10.1111/j.1476-5381.1988.tb11565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Featherstone R. L., Robinson C., Holgate S. T., Church M. K. Evidence for thromboxane receptor mediated contraction of guinea-pig and human airways in vitro by prostaglandin (PG) D2, 9 alpha,11 beta-PGF2 and PGF2 alpha. Naunyn Schmiedebergs Arch Pharmacol. 1990 May;341(5):439–443. doi: 10.1007/BF00176337. [DOI] [PubMed] [Google Scholar]
- Funk C. D., Furci L., FitzGerald G. A., Grygorczyk R., Rochette C., Bayne M. A., Abramovitz M., Adam M., Metters K. M. Cloning and expression of a cDNA for the human prostaglandin E receptor EP1 subtype. J Biol Chem. 1993 Dec 15;268(35):26767–26772. [PubMed] [Google Scholar]
- Furchgott R. F. The role of endothelium in the responses of vascular smooth muscle to drugs. Annu Rev Pharmacol Toxicol. 1984;24:175–197. doi: 10.1146/annurev.pa.24.040184.001135. [DOI] [PubMed] [Google Scholar]
- Furchgott R. F., Zawadzki J. V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980 Nov 27;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Giles H., Bolofo M. L., Lydford S. J., Martin G. R. A comparative study of the prostanoid receptor profile of 9 alpha 11 beta-prostaglandin F2 and prostaglandin D2. Br J Pharmacol. 1991 Oct;104(2):541–549. doi: 10.1111/j.1476-5381.1991.tb12465.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giles H., Leff P., Bolofo M. L., Kelly M. G., Robertson A. D. The classification of prostaglandin DP-receptors in platelets and vasculature using BW A868C, a novel, selective and potent competitive antagonist. Br J Pharmacol. 1989 Feb;96(2):291–300. doi: 10.1111/j.1476-5381.1989.tb11816.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haugen G., Hovig T. Studies of autacoid responsiveness and endothelium dependency in human umbilical arteries. Scand J Clin Lab Invest. 1992 May;52(3):141–149. doi: 10.3109/00365519209088778. [DOI] [PubMed] [Google Scholar]
- Hayashi S., Park M. K., Kuehl T. J. Relaxant and contractile responses to prostaglandins in premature, newborn and adult baboon cerebral arteries. J Pharmacol Exp Ther. 1985 Jun;233(3):628–635. [PubMed] [Google Scholar]
- Hirata M., Hayashi Y., Ushikubi F., Yokota Y., Kageyama R., Nakanishi S., Narumiya S. Cloning and expression of cDNA for a human thromboxane A2 receptor. Nature. 1991 Feb 14;349(6310):617–620. doi: 10.1038/349617a0. [DOI] [PubMed] [Google Scholar]
- Honda A., Sugimoto Y., Namba T., Watabe A., Irie A., Negishi M., Narumiya S., Ichikawa A. Cloning and expression of a cDNA for mouse prostaglandin E receptor EP2 subtype. J Biol Chem. 1993 Apr 15;268(11):7759–7762. [PubMed] [Google Scholar]
- Ignarro L. J., Buga G. M., Wood K. S., Byrns R. E., Chaudhuri G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9265–9269. doi: 10.1073/pnas.84.24.9265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa T., Cook D. I. Effects of K+ channel blockers on inwardly and outwardly rectifying whole-cell K+ currents in sheep parotid secretory cells. J Membr Biol. 1993 Apr;133(1):29–41. doi: 10.1007/BF00231875. [DOI] [PubMed] [Google Scholar]
- Jones R. L., Peesapati V., Wilson N. H. Antagonism of the thromboxane-sensitive contractile systems of the rabbit aorta, dog saphenous vein and guinea-pig trachea. Br J Pharmacol. 1982 Jul;76(3):423–438. doi: 10.1111/j.1476-5381.1982.tb09236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai Y., Ohhashi T. Prostaglandin F2 alpha-induced endothelium-dependent relaxation in isolated monkey cerebral arteries. Am J Physiol. 1991 May;260(5 Pt 2):H1538–H1543. doi: 10.1152/ajpheart.1991.260.5.H1538. [DOI] [PubMed] [Google Scholar]
- Lansman J. B. Endothelial mechanosensors. Going with the flow. Nature. 1988 Feb 11;331(6156):481–482. doi: 10.1038/331481a0. [DOI] [PubMed] [Google Scholar]
- Lawrence R. A., Jones R. L. Investigation of the prostaglandin E (EP-) receptor subtype mediating relaxation of the rabbit jugular vein. Br J Pharmacol. 1992 Apr;105(4):817–824. doi: 10.1111/j.1476-5381.1992.tb09063.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lückhoff A., Busse R. Activators of potassium channels enhance calcium influx into endothelial cells as a consequence of potassium currents. Naunyn Schmiedebergs Arch Pharmacol. 1990 Jul;342(1):94–99. doi: 10.1007/BF00178979. [DOI] [PubMed] [Google Scholar]
- Lückhoff A., Pohl U., Mülsch A., Busse R. Differential role of extra- and intracellular calcium in the release of EDRF and prostacyclin from cultured endothelial cells. Br J Pharmacol. 1988 Sep;95(1):189–196. doi: 10.1111/j.1476-5381.1988.tb16564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macphee C. H., Drummond A. H., Otto A. M., Jimenez de Asua L. Prostaglandin F2 alpha stimulates phosphatidylinositol turnover and increases the cellular content of 1,2-diacylglycerol in confluent resting Swiss 3T3 cells. J Cell Physiol. 1984 Apr;119(1):35–40. doi: 10.1002/jcp.1041190107. [DOI] [PubMed] [Google Scholar]
- Martin G. R., Bolofo M. L., Giles H. Inhibition of endothelium-dependent vasorelaxation by arginine analogues: a pharmacological analysis of agonist and tissue dependence. Br J Pharmacol. 1992 Mar;105(3):643–652. doi: 10.1111/j.1476-5381.1992.tb09033.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moncada S., Higgs A. The L-arginine-nitric oxide pathway. N Engl J Med. 1993 Dec 30;329(27):2002–2012. doi: 10.1056/NEJM199312303292706. [DOI] [PubMed] [Google Scholar]
- Moncada S., Palmer R. M., Higgs E. A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991 Jun;43(2):109–142. [PubMed] [Google Scholar]
- Nakao A., Watanabe T., Taniguchi S., Nakamura M., Honda Z., Shimizu T., Kurokawa K. Characterization of prostaglandin F2 alpha receptor of mouse 3T3 fibroblasts and its functional expression in Xenopus laevis oocytes. J Cell Physiol. 1993 May;155(2):257–264. doi: 10.1002/jcp.1041550206. [DOI] [PubMed] [Google Scholar]
- Namba T., Oida H., Sugimoto Y., Kakizuka A., Negishi M., Ichikawa A., Narumiya S. cDNA cloning of a mouse prostacyclin receptor. Multiple signaling pathways and expression in thymic medulla. J Biol Chem. 1994 Apr 1;269(13):9986–9992. [PubMed] [Google Scholar]
- Namba T., Sugimoto Y., Negishi M., Irie A., Ushikubi F., Kakizuka A., Ito S., Ichikawa A., Narumiya S. Alternative splicing of C-terminal tail of prostaglandin E receptor subtype EP3 determines G-protein specificity. Nature. 1993 Sep 9;365(6442):166–170. doi: 10.1038/365166a0. [DOI] [PubMed] [Google Scholar]
- Narumiya S., Hirata N., Namba T., Hayashi Y., Ushikubi F., Sugimoto Y., Negishi M., Ichikawa A. Structure and function of prostanoid receptors. J Lipid Mediat. 1993 Mar-Apr;6(1-3):155–161. [PubMed] [Google Scholar]
- Nelson M. T., Patlak J. B., Worley J. F., Standen N. B. Calcium channels, potassium channels, and voltage dependence of arterial smooth muscle tone. Am J Physiol. 1990 Jul;259(1 Pt 1):C3–18. doi: 10.1152/ajpcell.1990.259.1.C3. [DOI] [PubMed] [Google Scholar]
- Ogletree M. L., Harris D. N., Greenberg R., Haslanger M. F., Nakane M. Pharmacological actions of SQ 29,548, a novel selective thromboxane antagonist. J Pharmacol Exp Ther. 1985 Aug;234(2):435–441. [PubMed] [Google Scholar]
- Palmer R. M., Ferrige A. G., Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987 Jun 11;327(6122):524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Ralevic V., Mathie R. T., Alexander B., Burnstock G. NG-nitro-L-arginine methyl ester attenuates vasodilator responses to acetylcholine but enhances those to sodium nitroprusside. J Pharm Pharmacol. 1991 Dec;43(12):871–874. doi: 10.1111/j.2042-7158.1991.tb03199.x. [DOI] [PubMed] [Google Scholar]
- Rees D. D., Palmer R. M., Schulz R., Hodson H. F., Moncada S. Characterization of three inhibitors of endothelial nitric oxide synthase in vitro and in vivo. Br J Pharmacol. 1990 Nov;101(3):746–752. doi: 10.1111/j.1476-5381.1990.tb14151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regan J. W., Bailey T. J., Donello J. E., Pierce K. L., Pepperl D. J., Zhang D., Kedzie K. M., Fairbairn C. E., Bogardus A. M., Woodward D. F. Molecular cloning and expression of human EP3 receptors: evidence of three variants with differing carboxyl termini. Br J Pharmacol. 1994 Jun;112(2):377–385. doi: 10.1111/j.1476-5381.1994.tb13082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusko J., Tanzi F., van Breemen C., Adams D. J. Calcium-activated potassium channels in native endothelial cells from rabbit aorta: conductance, Ca2+ sensitivity and block. J Physiol. 1992 Sep;455:601–621. doi: 10.1113/jphysiol.1992.sp019318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K., Ozaki H., Karaki H. Differential effects of carbachol on cytosolic calcium levels in vascular endothelium and smooth muscle. J Pharmacol Exp Ther. 1990 Oct;255(1):114–119. [PubMed] [Google Scholar]
- Senior J., Sangha R., Baxter G. S., Marshall K., Clayton J. K. In vitro characterization of prostanoid FP-, DP-, IP- and TP-receptors on the non-pregnant human myometrium. Br J Pharmacol. 1992 Sep;107(1):215–221. doi: 10.1111/j.1476-5381.1992.tb14489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto Y., Hasumoto K., Namba T., Irie A., Katsuyama M., Negishi M., Kakizuka A., Narumiya S., Ichikawa A. Cloning and expression of a cDNA for mouse prostaglandin F receptor. J Biol Chem. 1994 Jan 14;269(2):1356–1360. [PubMed] [Google Scholar]
- Taylor S. G., Weston A. H. Endothelium-derived hyperpolarizing factor: a new endogenous inhibitor from the vascular endothelium. Trends Pharmacol Sci. 1988 Aug;9(8):272–274. doi: 10.1016/0165-6147(88)90003-x. [DOI] [PubMed] [Google Scholar]
- Toda N., Inoue S., Okamura T., Okunishi H. Mechanism underlying relaxations caused by prostaglandins and thromboxane A2 analog in isolated dog arteries. J Cardiovasc Pharmacol. 1988 Mar;11(3):354–362. doi: 10.1097/00005344-198803000-00014. [DOI] [PubMed] [Google Scholar]
- Woodward D. F., Burke J. A., Williams L. S., Palmer B. P., Wheeler L. A., Woldemussie E., Ruiz G., Chen J. Prostaglandin F2 alpha effects on intraocular pressure negatively correlate with FP-receptor stimulation. Invest Ophthalmol Vis Sci. 1989 Aug;30(8):1838–1842. [PubMed] [Google Scholar]
- Woodward D. F., Lawrence R. A. Identification of a single (FP) receptor associated with prostanoid-induced Ca2+ signals in Swiss 3T3 cells. Biochem Pharmacol. 1994 Apr 29;47(9):1567–1574. doi: 10.1016/0006-2952(94)90533-9. [DOI] [PubMed] [Google Scholar]


