Abstract
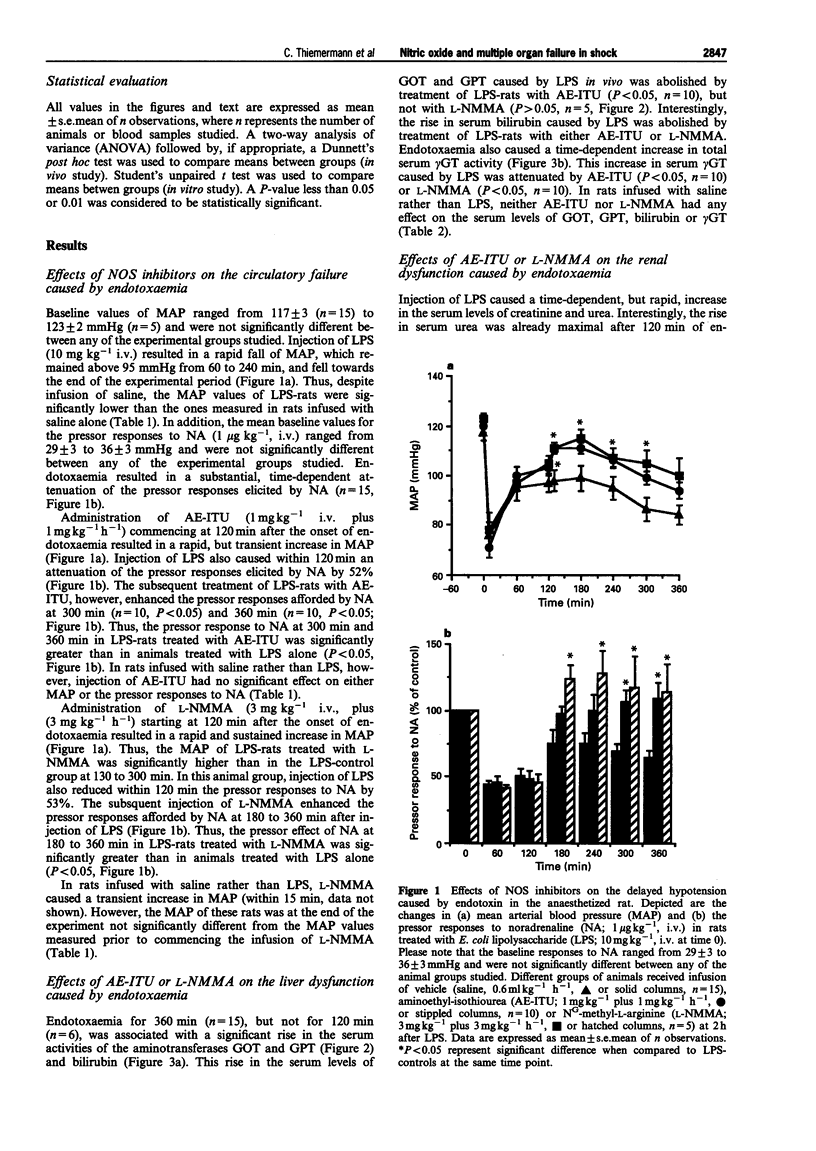
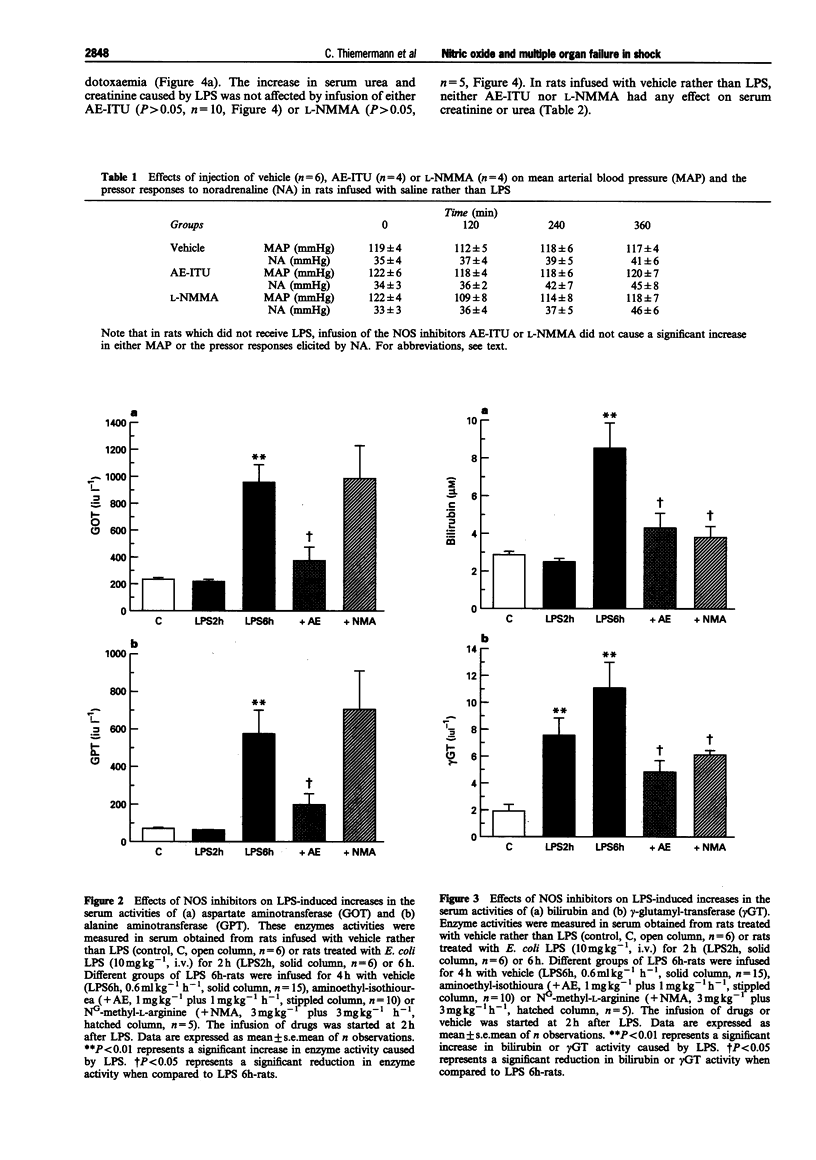
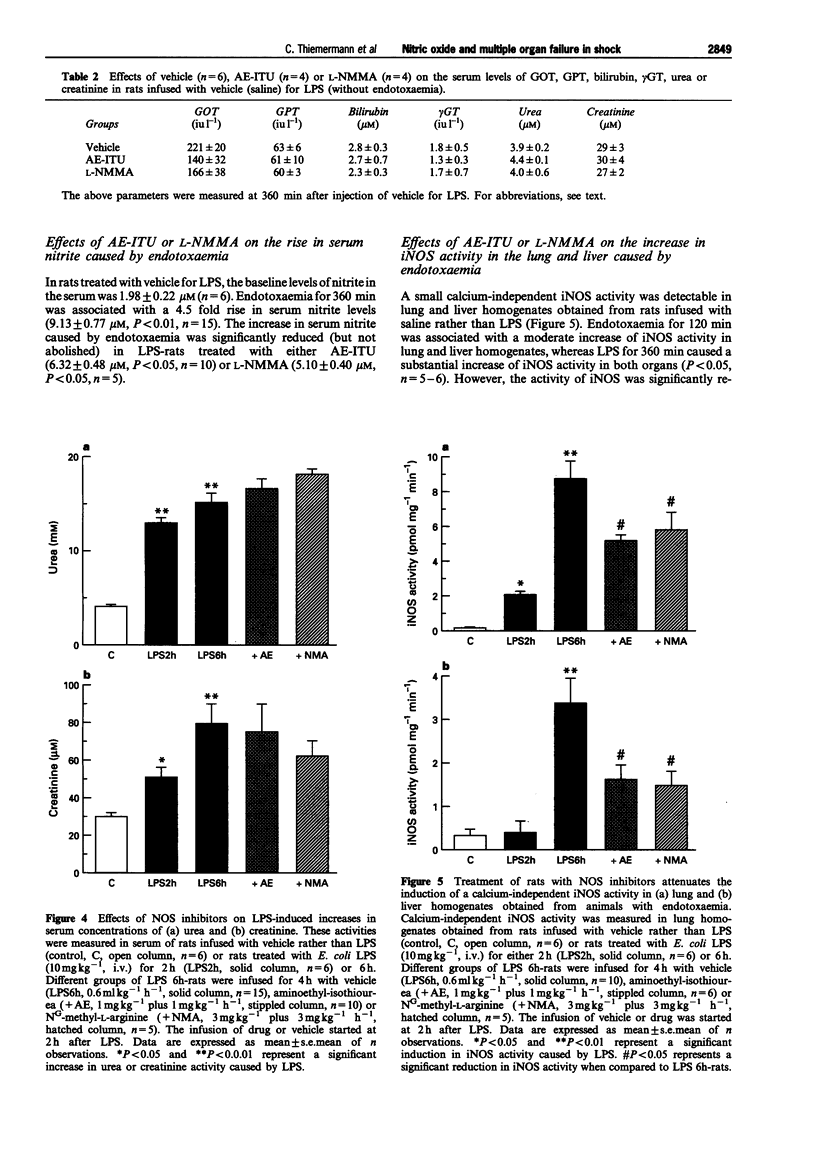
1. We have investigated whether (i) endotoxaemia caused by E. coli lipopolysaccharide in the anaesthetized rat causes a multiple organ dysfunction syndrome (MODS; e.g. circulatory failure, renal failure, liver failure), and (ii) an enhanced formation of nitric oxide (NO) due to induction of inducible NO synthase (iNOS) contributes to the MODS. In addition, this study elucidates the beneficial and adverse effects of aminoethyl-isothiourea (AE-ITU), a relatively selective inhibitor of iNOS activity, and NG-methyl-L-arginine (L-NMMA), a non-selective inhibitor of NOS activity on the MODS caused by endotoxaemia. 2. In the anaesthetized rat, LPS caused a fall in mean arterial blood pressure (MAP) from 117 +/- 3 mmHg (time 0) to 97 +/- 4 mmHg at 2 h (P < 0.05, n = 15) and 84 +/- 4 mmHg at 6 h (P < 0.05, n = 15). The pressor effect of noradrenaline (NA, 1 micrograms kg-1, i.v.) was also significantly reduced at 1 to 6 h after LPS (vascular hyporeactivity). Treatment of LPS-rats with AE-ITU (1 mg kg-1, i.v. plus 1 mg kg-1 h-1 starting at 2 h after LPS) caused only a transient rise in MAP, but significantly attenuated the delayed vascular hyporeactivity seen in LPS-rats. Infusion of L-NMMA (3 mg kg-1, i.v. plus 3 mg kg-1 h-1) caused a rapid and sustained rise in MAP and attenuated the delayed vascular hyporeactivity to NA. Neither AE-ITU nor L-NMMA had any effect on either MAP or the pressor effect elicited by NA in rats infused with saline rather than LPS. 3. Endotoxaemia for 6 h was associated with a significant rise in the serum levels of aspartate or alanine aminotransferase (i.e. GOT or GPT), gamma-glutamyl-transferase (gamma GT), and bilirubin, and hence, liver dysfunction. Treatment of LPS-rats with AE-ITU significantly attenuated this liver dysfunction (rise in GOT, GPT, gamma GT and bilirubin) (P < 0.05, n = 10). In contrast, L-NMMA reduced the increase in the serum levels of gamma GT and bilirubin, but not in GOT and GPT (n = 5). Injection of LPS also caused a time-dependent, but rapid (almost maximal at 2 h), increase in the serum levels of urea and creatinine, and hence, renal dysfunction. This renal dysfunction was not affected by either AE-ITU (n = 10) or L-NMMA (n = 5). In rats infused with saline rather than LPS, neither AE-ITU (n = 4) nor L-NMMA (n = 4) had any significant effect on the serum levels of GOT, GPT, gamma GT, bilirubin, creatinine or urea. 4. Endotoxaemia for 6 h resulted in a 4.5 fold rise in the serum levels of nitrite (9.13 +/- 0.77 microM, P < 0.01, n = 15), which was significantly reduced by treatment with AE-ITU (6.32 +/- 0.48 microM, P < 0.05, n = 10) or L-NMMA (5.10 +/- 0.40 microM, P < 0.05, n = 5). In addition, endotoxaemia for 6 h was also associated with a significant increase in iNOS activity in lung and liver homogenates, which was significantly reduced in lung or liver homogenates obtained from LPS-rats treated with either AE-ITU or L-NMMA. 5. Thus, AE-ITU or L-NMMA (i) inhibits iNOS activity in LPS-rats without causing a significant increase in MAP in rats infused with saline and, hence inhibition of endothelial NOS activity, and (ii) attenuates the delayed circulatory failure as well as the liver dysfunction caused by endotoxaemia in the rat. Thus, an enhanced formation of NO may contribute to the development of liver failure in endotoxic shock.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Billiar T. R., Curran R. D., Stuehr D. J., West M. A., Bentz B. G., Simmons R. L. An L-arginine-dependent mechanism mediates Kupffer cell inhibition of hepatocyte protein synthesis in vitro. J Exp Med. 1989 Apr 1;169(4):1467–1472. doi: 10.1084/jem.169.4.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cobb J. P., Natanson C., Hoffman W. D., Lodato R. F., Banks S., Koev C. A., Solomon M. A., Elin R. J., Hosseini J. M., Danner R. L. N omega-amino-L-arginine, an inhibitor of nitric oxide synthase, raises vascular resistance but increases mortality rates in awake canines challenged with endotoxin. J Exp Med. 1992 Oct 1;176(4):1175–1182. doi: 10.1084/jem.176.4.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran R. D., Ferrari F. K., Kispert P. H., Stadler J., Stuehr D. J., Simmons R. L., Billiar T. R. Nitric oxide and nitric oxide-generating compounds inhibit hepatocyte protein synthesis. FASEB J. 1991 Apr;5(7):2085–2092. doi: 10.1096/fasebj.5.7.1707021. [DOI] [PubMed] [Google Scholar]
- Deitch E. A. Multiple organ failure. Pathophysiology and potential future therapy. Ann Surg. 1992 Aug;216(2):117–134. doi: 10.1097/00000658-199208000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garvey E. P., Oplinger J. A., Tanoury G. J., Sherman P. A., Fowler M., Marshall S., Harmon M. F., Paith J. E., Furfine E. S. Potent and selective inhibition of human nitric oxide synthases. Inhibition by non-amino acid isothioureas. J Biol Chem. 1994 Oct 28;269(43):26669–26676. [PubMed] [Google Scholar]
- Green L. C., Ruiz de Luzuriaga K., Wagner D. A., Rand W., Istfan N., Young V. R., Tannenbaum S. R. Nitrate biosynthesis in man. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7764–7768. doi: 10.1073/pnas.78.12.7764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross S. S., Stuehr D. J., Aisaka K., Jaffe E. A., Levi R., Griffith O. W. Macrophage and endothelial cell nitric oxide synthesis: cell-type selective inhibition by NG-aminoarginine, NG-nitroarginine and NG-methylarginine. Biochem Biophys Res Commun. 1990 Jul 16;170(1):96–103. doi: 10.1016/0006-291x(90)91245-n. [DOI] [PubMed] [Google Scholar]
- Harbrecht B. G., Billiar T. R., Stadler J., Demetris A. J., Ochoa J., Curran R. D., Simmons R. L. Inhibition of nitric oxide synthesis during endotoxemia promotes intrahepatic thrombosis and an oxygen radical-mediated hepatic injury. J Leukoc Biol. 1992 Oct;52(4):390–394. doi: 10.1002/jlb.52.4.390. [DOI] [PubMed] [Google Scholar]
- Harbrecht B. G., Stadler J., Demetris A. J., Simmons R. L., Billiar T. R. Nitric oxide and prostaglandins interact to prevent hepatic damage during murine endotoxemia. Am J Physiol. 1994 Jun;266(6 Pt 1):G1004–G1010. doi: 10.1152/ajpgi.1994.266.6.G1004. [DOI] [PubMed] [Google Scholar]
- Hewett J. A., Roth R. A. The coagulation system, but not circulating fibrinogen, contributes to liver injury in rats exposed to lipopolysaccharide from gram-negative bacteria. J Pharmacol Exp Ther. 1995 Jan;272(1):53–62. [PubMed] [Google Scholar]
- Hibbs J. B., Jr, Taintor R. R., Vavrin Z. Macrophage cytotoxicity: role for L-arginine deiminase and imino nitrogen oxidation to nitrite. Science. 1987 Jan 23;235(4787):473–476. doi: 10.1126/science.2432665. [DOI] [PubMed] [Google Scholar]
- Hutcheson I. R., Whittle B. J., Boughton-Smith N. K. Role of nitric oxide in maintaining vascular integrity in endotoxin-induced acute intestinal damage in the rat. Br J Pharmacol. 1990 Dec;101(4):815–820. doi: 10.1111/j.1476-5381.1990.tb14163.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moncada S., Higgs A. The L-arginine-nitric oxide pathway. N Engl J Med. 1993 Dec 30;329(27):2002–2012. doi: 10.1056/NEJM199312303292706. [DOI] [PubMed] [Google Scholar]
- Morris S. M., Jr, Billiar T. R. New insights into the regulation of inducible nitric oxide synthesis. Am J Physiol. 1994 Jun;266(6 Pt 1):E829–E839. doi: 10.1152/ajpendo.1994.266.6.E829. [DOI] [PubMed] [Google Scholar]
- Rees D. D., Palmer R. M., Hodson H. F., Moncada S. A specific inhibitor of nitric oxide formation from L-arginine attenuates endothelium-dependent relaxation. Br J Pharmacol. 1989 Feb;96(2):418–424. doi: 10.1111/j.1476-5381.1989.tb11833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shultz P. J., Raij L. Endogenously synthesized nitric oxide prevents endotoxin-induced glomerular thrombosis. J Clin Invest. 1992 Nov;90(5):1718–1725. doi: 10.1172/JCI116045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southan G. J., Szabó C., Thiemermann C. Isothioureas: potent inhibitors of nitric oxide synthases with variable isoform selectivity. Br J Pharmacol. 1995 Jan;114(2):510–516. doi: 10.1111/j.1476-5381.1995.tb13256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadler J., Harbrecht B. G., Di Silvio M., Curran R. D., Jordan M. L., Simmons R. L., Billiar T. R. Endogenous nitric oxide inhibits the synthesis of cyclooxygenase products and interleukin-6 by rat Kupffer cells. J Leukoc Biol. 1993 Feb;53(2):165–172. doi: 10.1002/jlb.53.2.165. [DOI] [PubMed] [Google Scholar]
- Stamler J. S., Singel D. J., Loscalzo J. Biochemistry of nitric oxide and its redox-activated forms. Science. 1992 Dec 18;258(5090):1898–1902. doi: 10.1126/science.1281928. [DOI] [PubMed] [Google Scholar]
- Szabó C., Salzman A. L. Endogenous peroxynitrite is involved in the inhibition of mitochondrial respiration in immuno-stimulated J774.2 macrophages. Biochem Biophys Res Commun. 1995 Apr 17;209(2):739–743. doi: 10.1006/bbrc.1995.1561. [DOI] [PubMed] [Google Scholar]
- Szabó C., Southan G. J., Thiemermann C. Beneficial effects and improved survival in rodent models of septic shock with S-methylisothiourea sulfate, a potent and selective inhibitor of inducible nitric oxide synthase. Proc Natl Acad Sci U S A. 1994 Dec 20;91(26):12472–12476. doi: 10.1073/pnas.91.26.12472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiemermann C. The role of the L-arginine: nitric oxide pathway in circulatory shock. Adv Pharmacol. 1994;28:45–79. doi: 10.1016/s1054-3589(08)60493-7. [DOI] [PubMed] [Google Scholar]
- Thiemermann C., Wu C. C., Szabó C., Perretti M., Vane J. R. Role of tumour necrosis factor in the induction of nitric oxide synthase in a rat model of endotoxin shock. Br J Pharmacol. 1993 Sep;110(1):177–182. doi: 10.1111/j.1476-5381.1993.tb13789.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright C. E., Rees D. D., Moncada S. Protective and pathological roles of nitric oxide in endotoxin shock. Cardiovasc Res. 1992 Jan;26(1):48–57. doi: 10.1093/cvr/26.1.48. [DOI] [PubMed] [Google Scholar]


