Abstract
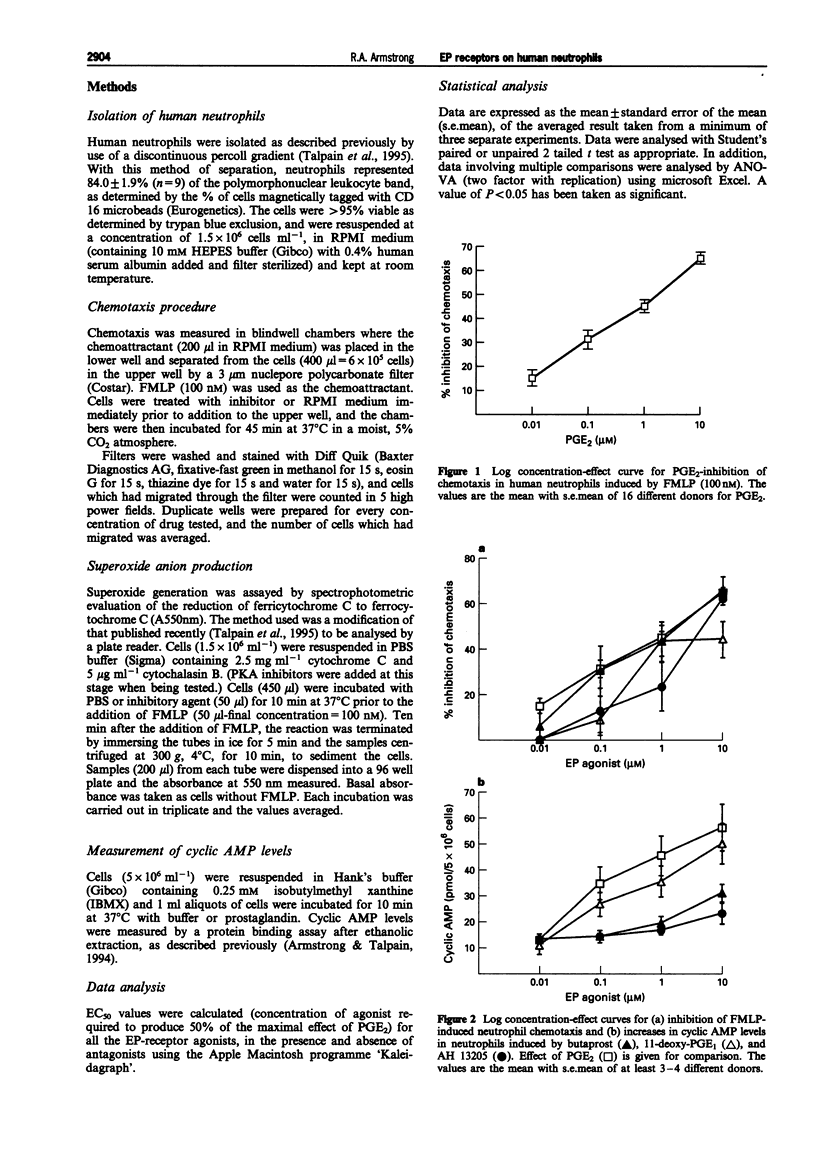
1. The aims of this study were to investigate the inhibitory effects of prostaglandin E2 (PGE2) on chemotaxis of N-formyl-methionyl-leucine-phenylalanine (FMLP)-stimulated human neutrophils, and to test the hypothesis that cyclic AMP is the second messenger involved. For this purpose, the inhibitory effect of selective EP agonists, and the modulatory effects of the adenylate cyclase inhibitor, SQ 22536, the protein kinase A (PKA) inhibitors H-89 and Rp-cAMPs, and the type IV phosphodiesterase (PDE) inhibitors, rolipram and Ro20-1724 have been examined. 2. Chemotaxis has been measured using blindwell chambers. When human neutrophils were stimulated with FMLP (100 nM), PGE2 inhibited chemotaxis in a concentration-dependent manner (0.01-10 microM), with an EC50 of 90 +/- 24.5 nM, a maximum effect ranging from 45-75% and a mean inhibition of 64.5 +/- 2.4%. 3. The EP2-receptor agonists, 11-deoxy PGE1, butaprost and AH 13205 also inhibited chemotaxis. The order of potency of these agonists was PGE2 > butaprost (EC50 = 106.4 +/- 63 nM) > 11-deoxy PGE1 (EC50 = 140.9 +/- 64.7 nM) > AH 13205 (EC50 = 1.58 +/- 0.73 microM). Correlation of the ability of EP2 agonists to increase cyclic AMP and to inhibit chemotaxis was poor (r = 0.38). 4. The IP agonist, cicaprost gave similar increases in cyclic AMP to those achieved with PGE2, yet produced 50% of the maximum inhibition of chemotaxis observed with PGE2. 5. Slight potentiation of the inhibitory effects of PGE2 after type IV PDE block was observed with rolipram (EC50 for PGE2 = 57.2 +/- 5.9; 35.2 +/- 6.8 nM) but not Ro20-1724 (EC50 for PGE2 = 216.0 +/- 59.7; 97.8 +/- 50.6 nM). Type IV PDE inhibitors are themselves potent inhibitors of chemotaxis with EC50 values of 23.0 +/- 2.3 and 73.6 +/- 10.3 nM for rolipram and Ro20-1724, respectively. 6. Inhibition of cyclic AMP production with the adenylate cyclase inhibitor SQ 22,536 (0.1 mM) failed to antagonize inhibition of chemotaxis by PGE2 (EC50s for PGE2 of 57.2 +/- 5.9 and 56.8 +/- 27.3 nM, in the absence and presence of SQ 22,536, respectively) despite a reduction in the increase in cyclic AMP induced by PGE2. 7. Inhibition of PKA with either H-89 (10 microM) or Rp cyclic AMPS (10 microM) similarly failed to antagonize inhibition of chemotaxis by PGE2; EC50 for PGE2 of 90 +/- 40 and PGE2 + H-89 60 +/- 17 nM; PGE2 216.0 +/- 58.7 and PGE2 + Rp cyclic AMP 76.9 +/- 14.7 nM. 8. Of the two PKA inhibitors tested, H-89 (10 microM) and Rp cyclic AMPS (10 microM), the more effective inhibitor of PGE2-induced inhibition of neutrophil superoxide anion generation was H-89 (EC50s for PGE2 were 0.36 +/- 0.1 and > 10 microM, respectively). We have previously shown this to be a cyclic AMP-dependent effect of PGE2. 9. Confirmation of block of PKA by H-89 was suggested by the finding that H-89 blocked inhibition of superoxide anion generation observed with the type IV PDE inhibitors rolipram and Ro20-1724; EC50s of 12.9 +/- 8.9 nM for rolipram alone and rolipram+H-89 > 1 microM; Ro20-1724 alone 59.5 +/- 28.1 nM and Ro20-1724 + H-89 > 1 microM. 10. The results suggest that inhibition of chemotaxis by PGE2 and EP2 agonists is not mediated by increased neutrophil cyclic AMP levels.
Full text
PDF
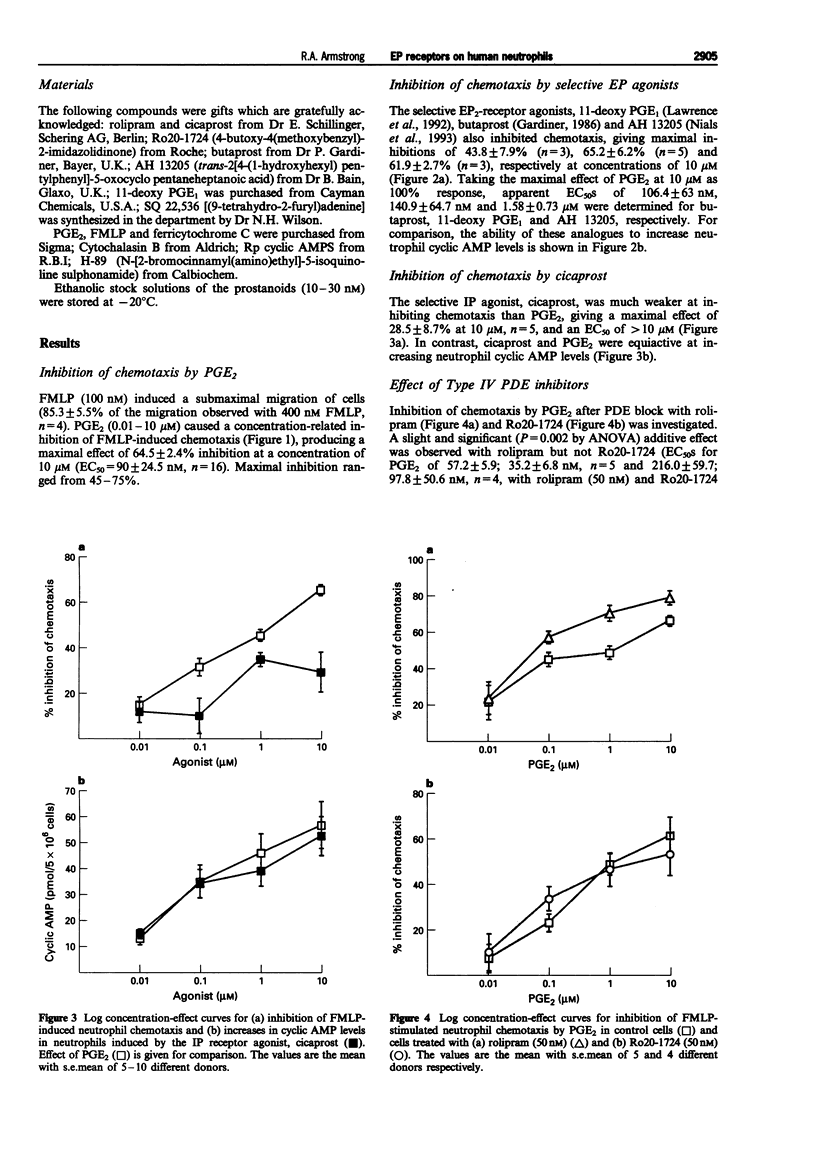

Selected References
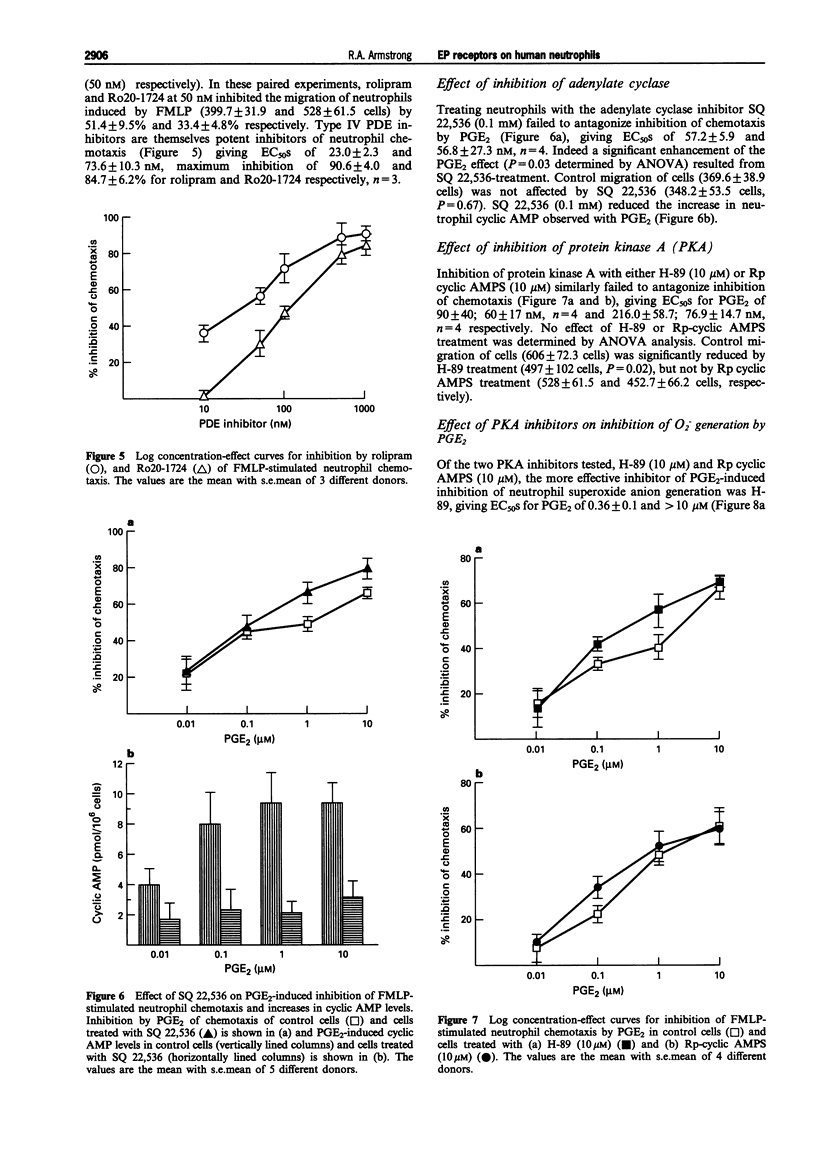
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong R. A., Talpain E. Comparison of the prostaglandin E (EP) receptor of human neutrophils and HL-60 cells differentiated with DMSO. Prostaglandins. 1994 Oct;48(4):221–234. doi: 10.1016/0090-6980(94)90009-4. [DOI] [PubMed] [Google Scholar]
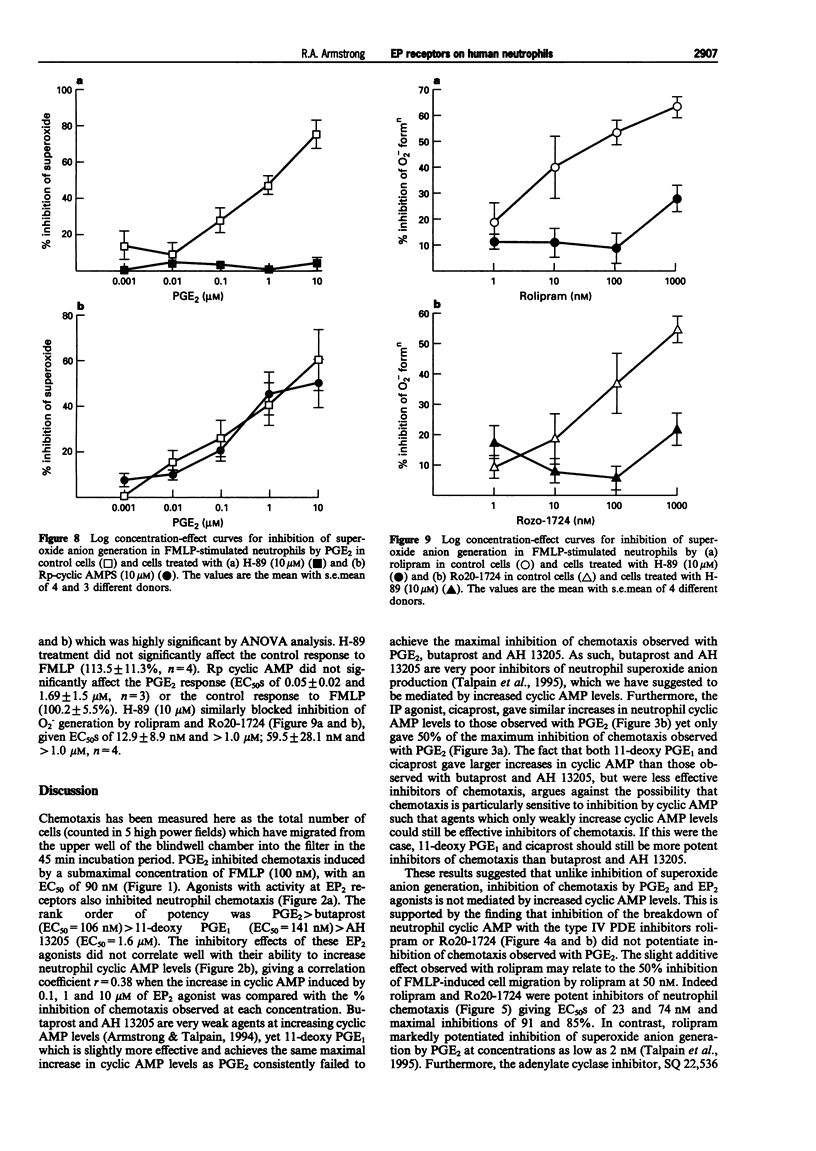
- BOYDEN S. The chemotactic effect of mixtures of antibody and antigen on polymorphonuclear leucocytes. J Exp Med. 1962 Mar 1;115:453–466. doi: 10.1084/jem.115.3.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belenky S. N., Robbins R. A., Rennard S. I., Gossman G. L., Nelson K. J., Rubinstein I. Inhibitors of nitric oxide synthase attenuate human neutrophil chemotaxis in vitro. J Lab Clin Med. 1993 Oct;122(4):388–394. [PubMed] [Google Scholar]
- Gardiner P. J. Characterization of prostanoid relaxant/inhibitory receptors (psi) using a highly selective agonist, TR4979. Br J Pharmacol. 1986 Jan;87(1):45–56. doi: 10.1111/j.1476-5381.1986.tb10155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geilen C. C., Wieprecht M., Wieder T., Reutter W. A selective inhibitor of cyclic AMP-dependent protein kinase, N-[2-bromocinnamyl(amino)ethyl]-5-isoquinolinesulfonamide (H-89), inhibits phosphatidylcholine biosynthesis in HeLa cells. FEBS Lett. 1992 Sep 14;309(3):381–384. doi: 10.1016/0014-5793(92)80811-t. [DOI] [PubMed] [Google Scholar]
- Harris D. N., Asaad M. M., Phillips M. B., Goldenberg H. J., Antonaccio M. J. Inhibition of adenylate cyclase in human blood platelets by 9-substituted adenine derivatives. J Cyclic Nucleotide Res. 1979;5(2):125–134. [PubMed] [Google Scholar]
- Hecker G., Ney P., Schrör K. Cytotoxic enzyme release and oxygen centered radical formation in human neutrophils are selectively inhibited by E-type prostaglandins but not by PGI2. Naunyn Schmiedebergs Arch Pharmacol. 1990 Apr;341(4):308–315. doi: 10.1007/BF00180656. [DOI] [PubMed] [Google Scholar]
- Lawrence R. A., Jones R. L., Wilson N. H. Characterization of receptors involved in the direct and indirect actions of prostaglandins E and I on the guinea-pig ileum. Br J Pharmacol. 1992 Feb;105(2):271–278. doi: 10.1111/j.1476-5381.1992.tb14245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musgrave I. F., Genieser H. G., Maronde E., Seifert R. Preparations of Rp-cyclic adenosine 3',5'-phosphorothioate (Rp-cAMPS) can contain biologically active amounts of adenosine. FEBS Lett. 1993 Mar 8;318(3):227–230. doi: 10.1016/0014-5793(93)80517-x. [DOI] [PubMed] [Google Scholar]
- Ney P., Schrör K. PGD2 and its mimetic ZK 110.841 are potent inhibitors of receptor-mediated activation of human neutrophils. Eicosanoids. 1991;4(1):21–28. [PubMed] [Google Scholar]
- Norgauer J., Kownatzki E., Seifert R., Aktories K. Botulinum C2 toxin ADP-ribosylates actin and enhances O2- production and secretion but inhibits migration of activated human neutrophils. J Clin Invest. 1988 Oct;82(4):1376–1382. doi: 10.1172/JCI113741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schudt C., Winder S., Forderkunz S., Hatzelmann A., Ullrich V. Influence of selective phosphodiesterase inhibitors on human neutrophil functions and levels of cAMP and Cai. Naunyn Schmiedebergs Arch Pharmacol. 1991 Dec;344(6):682–690. doi: 10.1007/BF00174752. [DOI] [PubMed] [Google Scholar]
- Talpain E., Armstrong R. A., Coleman R. A., Vardey C. J. Characterization of the PGE receptor subtype mediating inhibition of superoxide production in human neutrophils. Br J Pharmacol. 1995 Apr;114(7):1459–1465. doi: 10.1111/j.1476-5381.1995.tb13370.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thelen M., Dewald B., Baggiolini M. Neutrophil signal transduction and activation of the respiratory burst. Physiol Rev. 1993 Oct;73(4):797–821. doi: 10.1152/physrev.1993.73.4.797. [DOI] [PubMed] [Google Scholar]
- Tranquillo R. T., Lauffenburger D. A., Zigmond S. H. A stochastic model for leukocyte random motility and chemotaxis based on receptor binding fluctuations. J Cell Biol. 1988 Feb;106(2):303–309. doi: 10.1083/jcb.106.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Haastert P. J., Van Driel R., Jastorff B., Baraniak J., Stec W. J., De Wit R. J. Competitive cAMP antagonists for cAMP-receptor proteins. J Biol Chem. 1984 Aug 25;259(16):10020–10024. [PubMed] [Google Scholar]
- Wilkinson P. C. Chemotaxis and chemokinesis: confusion about definitions. J Immunol Methods. 1988 May 25;110(1):143–149. doi: 10.1016/0022-1759(88)90094-4. [DOI] [PubMed] [Google Scholar]
- Wong K., Freund K. Inhibition of the n-formylmethionyl-leucyl-phenylalanine induced respiratory burst in human neutrophils by adrenergic agonists and prostaglandins of the E series. Can J Physiol Pharmacol. 1981 Sep;59(9):915–920. doi: 10.1139/y81-141. [DOI] [PubMed] [Google Scholar]
- Wyatt T. A., Lincoln T. M., Pryzwansky K. B. Vimentin is transiently co-localized with and phosphorylated by cyclic GMP-dependent protein kinase in formyl-peptide-stimulated neutrophils. J Biol Chem. 1991 Nov 5;266(31):21274–21280. [PubMed] [Google Scholar]


