Abstract
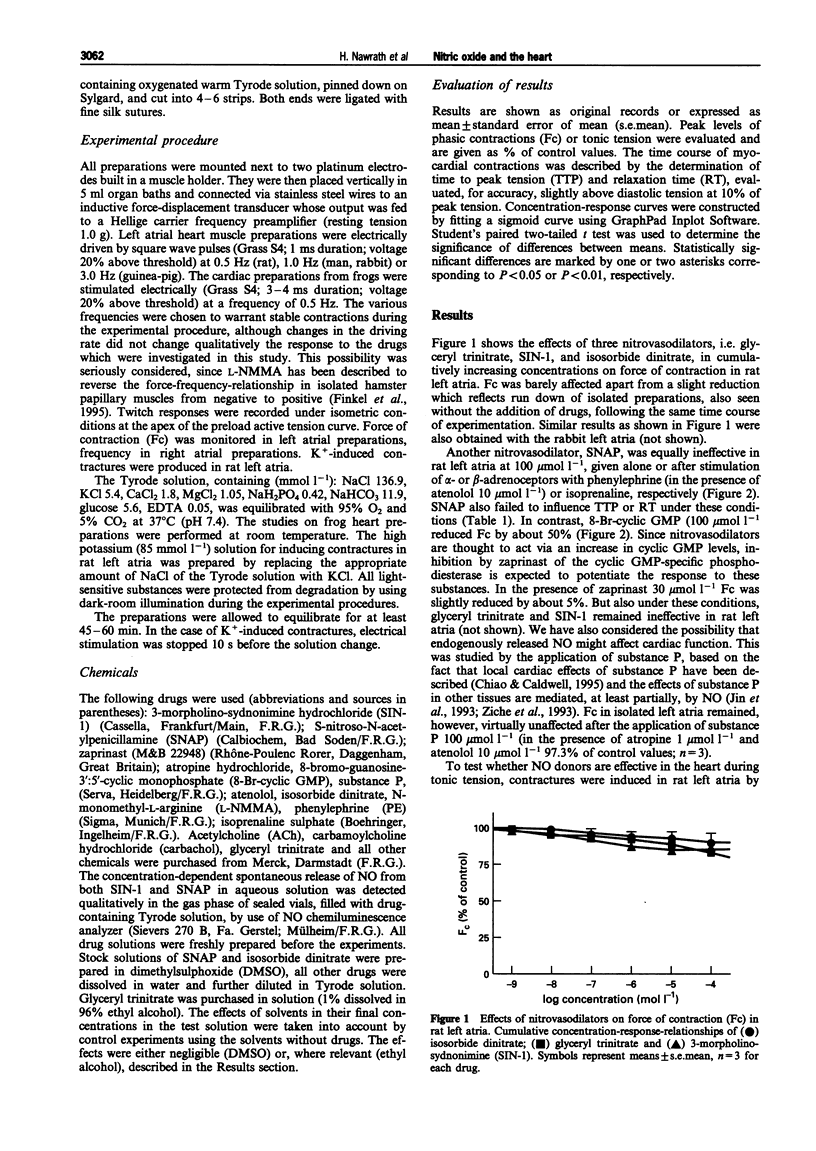
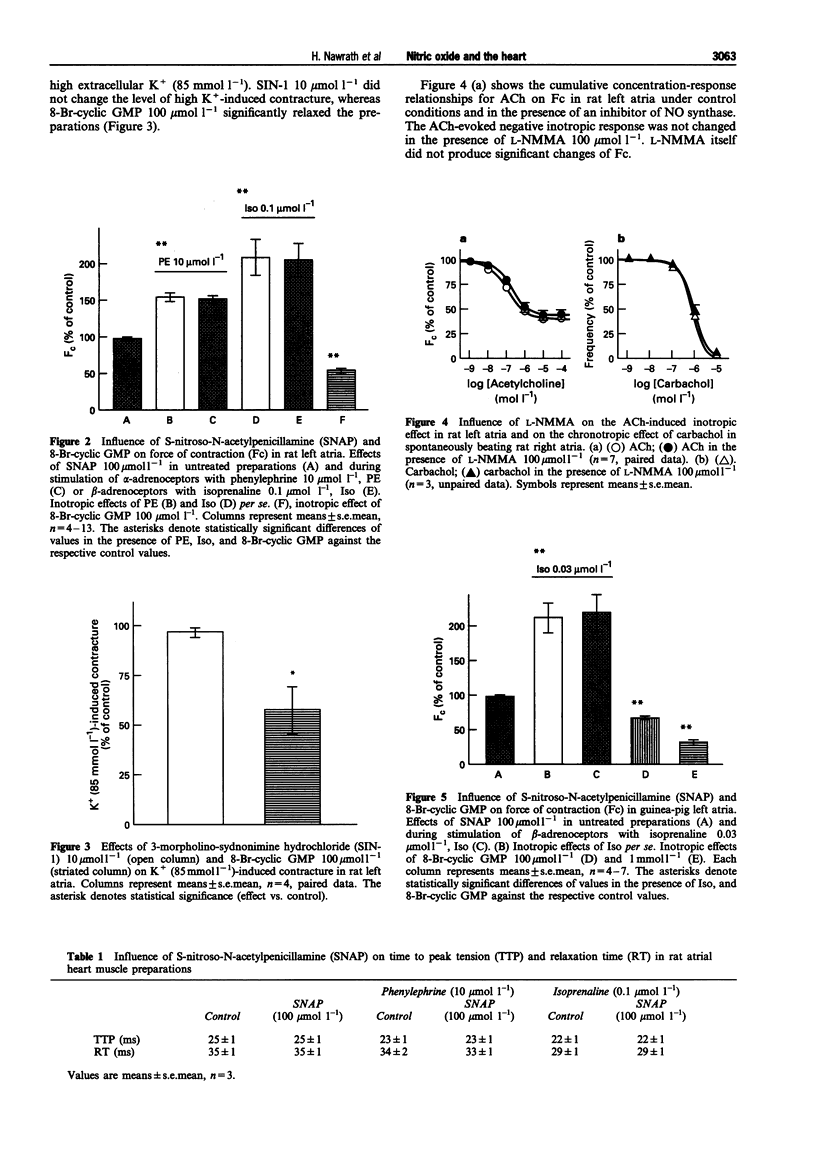
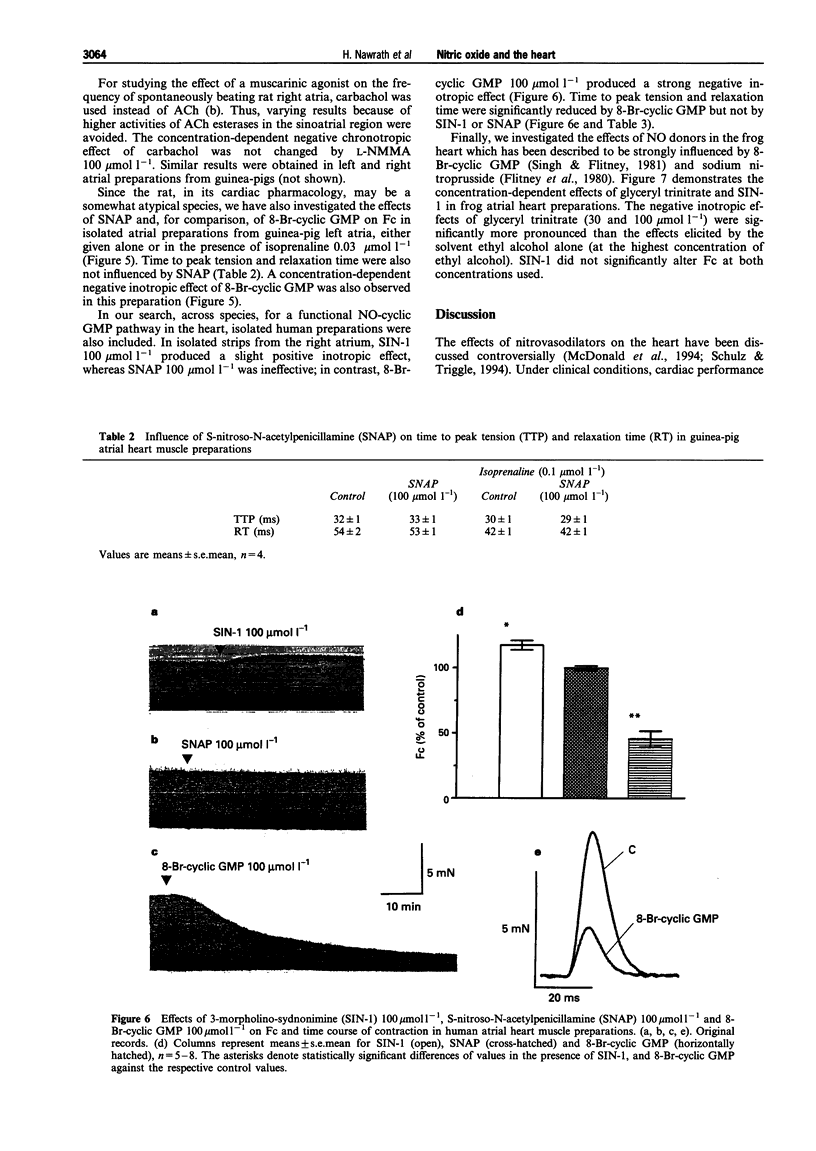
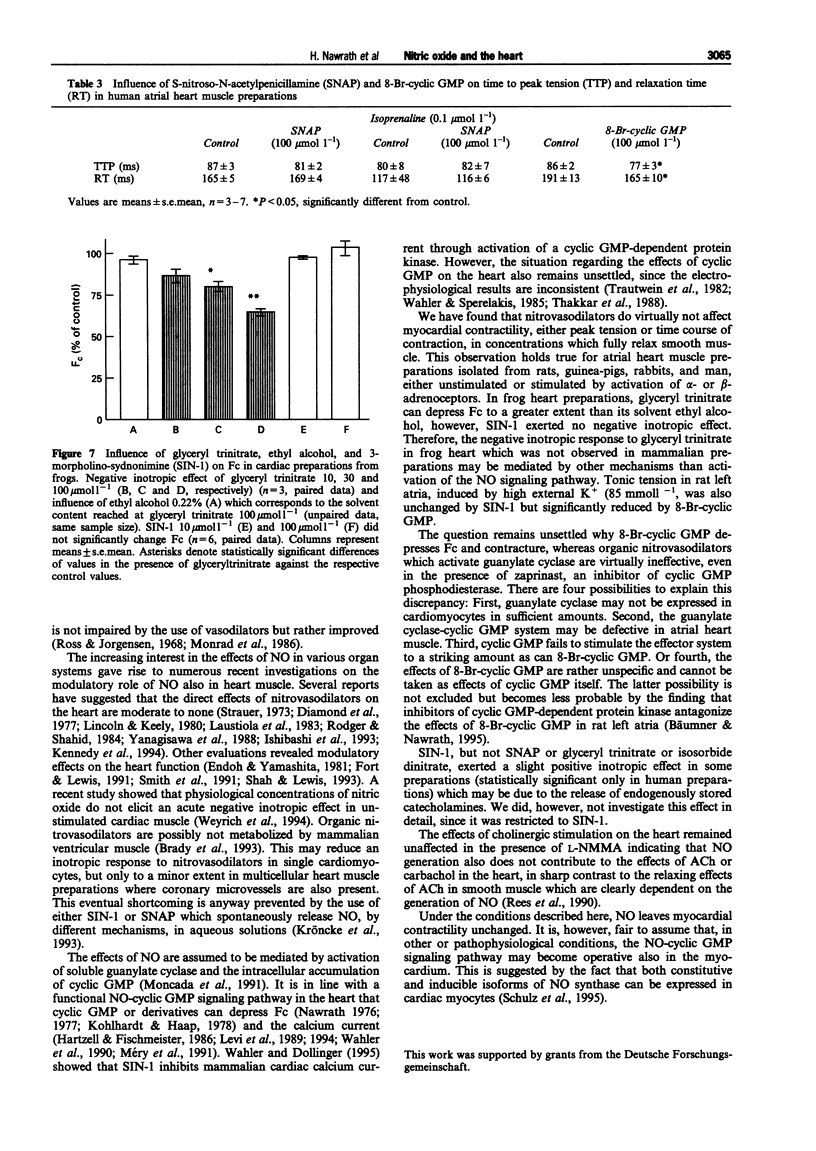
1. This study was performed to determine whether nitric oxide (NO) has direct effects on force of contraction (Fc) in atrial myocardium from rats, rabbits, guinea-pigs, frogs, and man. 2. Glyceryl trinitrate, isosorbide dinitrate, 3-morpholino-sydnonimine hydrochloride (SIN-1), and S-nitroso-N-acetylpenicillamine (SNAP) did not significantly reduce Fc in the various preparations investigated, either given alone or after stimulation of alpha- or beta-adrenoceptors. 3. SNAP did not change the time course of contractions in rat, guinea-pig and human preparations. 4. 8-Bromo-guanosine-3':5'-cyclic monophosphate (8-Br-cyclic GMP) produced a negative inotropic effect in rat, guinea-pig and human atrial preparations and shortened time to peak tension and relaxation time in human preparations. 5. High K+ (85 mmol l-1)-induced contracture in rat heart muscle was reduced by 8-Br-cyclic GMP but not by SIN-1. 6. N-monomethyl-L-arginine (L-NMMA), an inhibitor of NO synthase, failed to influence muscarinic effects on Fc or frequency from rat and guinea-pig hearts. 7. We conclude that NO, under the experimental conditions described here, has no direct effects on the heart, although cyclic GMP may be involved in the regulation of myocardial contraction.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brady A. J., Warren J. B., Poole-Wilson P. A., Williams T. J., Harding S. E. Nitric oxide attenuates cardiac myocyte contraction. Am J Physiol. 1993 Jul;265(1 Pt 2):H176–H182. doi: 10.1152/ajpheart.1993.265.1.H176. [DOI] [PubMed] [Google Scholar]
- Bäumner D., Nawrath H. Effects of inhibitors of cGMP-dependent protein kinase in atrial heart and aortic smooth muscle from rats. Eur J Pharmacol. 1995 Feb 6;273(3):295–298. doi: 10.1016/0014-2999(94)00752-s. [DOI] [PubMed] [Google Scholar]
- Chiao H., Caldwell R. W. Local cardiac effects of substance P: roles of acetylcholine and noradrenaline. Br J Pharmacol. 1995 Jan;114(2):283–288. doi: 10.1111/j.1476-5381.1995.tb13224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond J., Ten Eick R. E., Trapani A. J. Are increases in cyclic GMP levels responsible for the negative inotropic effects of acetylcholine in the heart? Biochem Biophys Res Commun. 1977 Dec 7;79(3):912–918. doi: 10.1016/0006-291x(77)91197-4. [DOI] [PubMed] [Google Scholar]
- Dinerman J. L., Lowenstein C. J., Snyder S. H. Molecular mechanisms of nitric oxide regulation. Potential relevance to cardiovascular disease. Circ Res. 1993 Aug;73(2):217–222. doi: 10.1161/01.res.73.2.217. [DOI] [PubMed] [Google Scholar]
- Endoh M., Yamashita S. Differential responses to carbachol, sodium nitroprusside and 8-bromo-guanosine 3',5'-monophosphate of canine atrial and ventricular muscle. Br J Pharmacol. 1981 Jun;73(2):393–399. doi: 10.1111/j.1476-5381.1981.tb10434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkel M. S., Oddis C. V., Jacob T. D., Watkins S. C., Hattler B. G., Simmons R. L. Negative inotropic effects of cytokines on the heart mediated by nitric oxide. Science. 1992 Jul 17;257(5068):387–389. doi: 10.1126/science.1631560. [DOI] [PubMed] [Google Scholar]
- Finkel M. S., Oddis C. V., Mayer O. H., Hattler B. G., Simmons R. L. Nitric oxide synthase inhibitor alters papillary muscle force-frequency relationship. J Pharmacol Exp Ther. 1995 Feb;272(2):945–952. [PubMed] [Google Scholar]
- Girolami J. P., Bascands J. L., Pécher C., Berlan M., Montastruc J. L., Montastruc P. Yohimbine increases submaxillary kallikrein release into the saliva in dogs: evidence for alpha 2-adrenoceptor-mediated inhibition of cholinergic pathways. Br J Pharmacol. 1991 Feb;102(2):351–354. doi: 10.1111/j.1476-5381.1991.tb12177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartzell H. C., Fischmeister R. Opposite effects of cyclic GMP and cyclic AMP on Ca2+ current in single heart cells. Nature. 1986 Sep 18;323(6085):273–275. doi: 10.1038/323273a0. [DOI] [PubMed] [Google Scholar]
- Ishibashi T., Hamaguchi M., Kato K., Kawada T., Ohta H., Sasage H., Imai S. Relationship between myoglobin contents and increases in cyclic GMP produced by glyceryl trinitrate and nitric oxide in rabbit aorta, right atrium and papillary muscle. Naunyn Schmiedebergs Arch Pharmacol. 1993 May;347(5):553–561. doi: 10.1007/BF00166750. [DOI] [PubMed] [Google Scholar]
- Jin J. G., Misra S., Grider J. R., Makhlouf G. M. Functional difference between SP and NKA: relaxation of gastric muscle by SP is mediated by VIP and NO. Am J Physiol. 1993 Apr;264(4 Pt 1):G678–G685. doi: 10.1152/ajpgi.1993.264.4.G678. [DOI] [PubMed] [Google Scholar]
- Kennedy R. H., Hicks K. K., Brian J. E., Jr, Seifen E. Nitric oxide has no chronotropic effect in right atria isolated from rat heart. Eur J Pharmacol. 1994 Apr 1;255(1-3):149–156. doi: 10.1016/0014-2999(94)90093-0. [DOI] [PubMed] [Google Scholar]
- Kohlhardt M., Haap K. 8-bromo-guanosine-3',5' -monophosphate mimics the effect of acetylcholine on slow response action potential and contractile force in mammalian atrial myocardium. J Mol Cell Cardiol. 1978 Jun;10(6):573–586. doi: 10.1016/0022-2828(78)90015-9. [DOI] [PubMed] [Google Scholar]
- Kröncke K. D., Brenner H. H., Rodriguez M. L., Etzkorn K., Noack E. A., Kolb H., Kolb-Bachofen V. Pancreatic islet cells are highly susceptible towards the cytotoxic effects of chemically generated nitric oxide. Biochim Biophys Acta. 1993 Sep 8;1182(2):221–229. doi: 10.1016/0925-4439(93)90144-p. [DOI] [PubMed] [Google Scholar]
- Laustiola K., Vuorinen P., Vapaatalo H., Metsä-Ketelä T. Sodium nitroprusside inhibits lactate formation in rat atria: is cyclic GMP involved? Acta Pharmacol Toxicol (Copenh) 1983 Mar;52(3):195–200. doi: 10.1111/j.1600-0773.1983.tb01085.x. [DOI] [PubMed] [Google Scholar]
- Levi R. C., Alloatti G., Fischmeister R. Cyclic GMP regulates the Ca-channel current in guinea pig ventricular myocytes. Pflugers Arch. 1989 Apr;413(6):685–687. doi: 10.1007/BF00581823. [DOI] [PubMed] [Google Scholar]
- Levi R. C., Alloatti G., Penna C., Gallo M. P. Guanylate-cyclase-mediated inhibition of cardiac ICa by carbachol and sodium nitroprusside. Pflugers Arch. 1994 Mar;426(5):419–426. doi: 10.1007/BF00388305. [DOI] [PubMed] [Google Scholar]
- Lincoln T. M., Keely S. L. Effects of acetylcholine and nitroprusside on cGMP-dependent protein kinase in the perfused rat heart. J Cyclic Nucleotide Res. 1980;6(2):83–91. [PubMed] [Google Scholar]
- McDonald T. F., Pelzer S., Trautwein W., Pelzer D. J. Regulation and modulation of calcium channels in cardiac, skeletal, and smooth muscle cells. Physiol Rev. 1994 Apr;74(2):365–507. doi: 10.1152/physrev.1994.74.2.365. [DOI] [PubMed] [Google Scholar]
- Moncada S., Palmer R. M., Higgs E. A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991 Jun;43(2):109–142. [PubMed] [Google Scholar]
- Méry P. F., Lohmann S. M., Walter U., Fischmeister R. Ca2+ current is regulated by cyclic GMP-dependent protein kinase in mammalian cardiac myocytes. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1197–1201. doi: 10.1073/pnas.88.4.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawrath H. Cyclic AMP and cyclic GMP may play opposing roles in influencing force of contraction in mammalian myocardium. Nature. 1976 Aug 5;262(5568):509–511. doi: 10.1038/262509b0. [DOI] [PubMed] [Google Scholar]
- Nawrath H. Does cyclic GMP mediate the negative inotropic effect of acetylcholine in the heart? Nature. 1977 May 5;267(5606):72–74. doi: 10.1038/267072a0. [DOI] [PubMed] [Google Scholar]
- Rapoport R. M., Draznin M. B., Murad F. Endothelium-dependent relaxation in rat aorta may be mediated through cyclic GMP-dependent protein phosphorylation. Nature. 1983 Nov 10;306(5939):174–176. doi: 10.1038/306174a0. [DOI] [PubMed] [Google Scholar]
- Rees D. D., Palmer R. M., Schulz R., Hodson H. F., Moncada S. Characterization of three inhibitors of endothelial nitric oxide synthase in vitro and in vivo. Br J Pharmacol. 1990 Nov;101(3):746–752. doi: 10.1111/j.1476-5381.1990.tb14151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodger I. W., Shahid M. Forskolin, cyclic nucleotides and positive inotropism in isolated papillary muscles of the rabbit. Br J Pharmacol. 1984 Jan;81(1):151–159. doi: 10.1111/j.1476-5381.1984.tb10755.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross G., Jorgensen C. Effects of iproveratril and nitroglycerin in the heart and coronary circulation of dogs. Am Heart J. 1968 Jul;76(1):74–78. doi: 10.1016/0002-8703(68)90297-4. [DOI] [PubMed] [Google Scholar]
- Schulz R., Panas D. L., Catena R., Moncada S., Olley P. M., Lopaschuk G. D. The role of nitric oxide in cardiac depression induced by interleukin-1 beta and tumour necrosis factor-alpha. Br J Pharmacol. 1995 Jan;114(1):27–34. doi: 10.1111/j.1476-5381.1995.tb14901.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz R., Triggle C. R. Role of NO in vascular smooth muscle and cardiac muscle function. Trends Pharmacol Sci. 1994 Jul;15(7):255–259. doi: 10.1016/0165-6147(94)90321-2. [DOI] [PubMed] [Google Scholar]
- Singh J., Flitney F. W. Inotropic responses of the frog ventricle to dibutyryl cyclic AMP and 8-bromo cyclic GMP and related changes in endogenous cyclic nucleotide levels. Biochem Pharmacol. 1981 Jun 15;30(12):1475–1481. doi: 10.1016/0006-2952(81)90370-1. [DOI] [PubMed] [Google Scholar]
- Smith J. A., Shah A. M., Lewis M. J. Factors released from endocardium of the ferret and pig modulate myocardial contraction. J Physiol. 1991 Aug;439:1–14. doi: 10.1113/jphysiol.1991.sp018653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauer B. E. Der Mechanismus der Nitroglyzerinwirkung vom Aspekt der Myokardkontraktilität. Tierexperimentelle Studien sowie Untersuchungen am isolierten menschlicyen Ventrikelmyokard. Z Kardiol. 1973 Feb;62(2):97–113. [PubMed] [Google Scholar]
- Thakkar J., Tang S. B., Sperelakis N., Wahler G. M. Inhibition of cardiac slow action potentials by 8-bromo-cyclic GMP occurs independent of changes in cyclic AMP levels. Can J Physiol Pharmacol. 1988 Aug;66(8):1092–1095. doi: 10.1139/y88-178. [DOI] [PubMed] [Google Scholar]
- Trautwein W., Taniguchi J., Noma A. The effect of intracellular cyclic nucleotides and calcium on the action potential and acetylcholine response of isolated cardiac cells. Pflugers Arch. 1982 Feb;392(4):307–314. doi: 10.1007/BF00581624. [DOI] [PubMed] [Google Scholar]
- Wahler G. M., Dollinger S. J. Nitric oxide donor SIN-1 inhibits mammalian cardiac calcium current through cGMP-dependent protein kinase. Am J Physiol. 1995 Jan;268(1 Pt 1):C45–C54. doi: 10.1152/ajpcell.1995.268.1.C45. [DOI] [PubMed] [Google Scholar]
- Wahler G. M., Rusch N. J., Sperelakis N. 8-Bromo-cyclic GMP inhibits the calcium channel current in embryonic chick ventricular myocytes. Can J Physiol Pharmacol. 1990 Apr;68(4):531–534. doi: 10.1139/y90-076. [DOI] [PubMed] [Google Scholar]
- Wahler G. M., Sperelakis N. Intracellular injection of cyclic GMP depresses cardiac slow action potentials. J Cyclic Nucleotide Protein Phosphor Res. 1985;10(1):83–95. [PubMed] [Google Scholar]
- Weyrich A. S., Ma X. L., Buerke M., Murohara T., Armstead V. E., Lefer A. M., Nicolas J. M., Thomas A. P., Lefer D. J., Vinten-Johansen J. Physiological concentrations of nitric oxide do not elicit an acute negative inotropic effect in unstimulated cardiac muscle. Circ Res. 1994 Oct;75(4):692–700. doi: 10.1161/01.res.75.4.692. [DOI] [PubMed] [Google Scholar]
- Yanagisawa T., Hashimoto H., Taira N. The negative inotropic effect of nicorandil is independent of cyclic GMP changes: a comparison with pinacidil and cromakalim in canine atrial muscle. Br J Pharmacol. 1988 Oct;95(2):393–398. doi: 10.1111/j.1476-5381.1988.tb11658.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziche M., Morbidelli L., Parenti A., Amerini S., Granger H. J., Maggi C. A. Substance P increases cyclic GMP levels on coronary postcapillary venular endothelial cells. Life Sci. 1993;53(14):PL229–PL234. doi: 10.1016/0024-3205(93)90556-i. [DOI] [PubMed] [Google Scholar]


