Abstract
1. The perforated patch and conventional whole-cell recording techniques were used to study the action of flufenamic, mefenamic and niflumic acid on calcium-activated chloride and potassium currents in rabbit portal vein smooth muscle cells. 2. In K-conditions at a holding potential of -77 mV flufenamic acid and mefenamic acid decreased the amplitude of spontaneous transient inward currents (STICs, calcium-activated chloride currents, ICl(Ca)) in a concentration-dependent manner. The potency sequence was niflumic > flufenamic > mefenamic acid. 3. At -77 mV 1 x 10(-5) M flufenamic acid increased the STIC exponential decay time constant (tau). At higher concentrations the STIC decay was described by 2 exponentials with an initial decay (tau f) faster than the control tau value and a second exponential (tau s) which had a time constant slower than the control tau value. Low concentrations of mefenamic acid had no effect or decreased the tau value whereas in higher concentrations biphasic currents were recorded. 4. In K-free conditions the inhibitory effect of both flufenamic and mefenamic acid on STIC amplitude was greater at +50 mV compared to -50 mV, showing that the effect of these agents was voltage-dependent. 5. In cells held at 0 mV in K-containing conditions the fenamates reduced both the frequency and amplitude of spontaneous transient outward currents (STOCs, calcium-activated potassium currents, IK(Ca)). The concentration range to produce these effects was higher than that to decrease STIC amplitude and the potency sequence was flufenamic > niflumic > or = mefenamic acid. 6. All these compounds in concentrations greater than 5 x 10(-5) M evoked a 'noisy' potassium current at 0 mV which reached a maximum after approximately 3 min. This current was readily reversible on washout of the drug and could be elicited several times in the same cell. The current-voltage relationship of the fenamate-evoked current exhibited pronounced outward rectification characteristic of IK(Ca). 7. The current evoked by 2 x 10(-4) M flufenamic acid and 5 x 10(-4) M niflumic acid was not affected by 1 x 10(-5) M glibenclamide but was markedly inhibited by 1 x 10(-3) M tetraethylammonium. Furthermore, large currents were activated by flufenamic and niflumic acid in the presence of caffeine and cyclopiazonic acid (an inhibitor of the sarcoplasmic reticulum Ca-ATPase) to deplete intracellular Ca-stores. 8. Conventional whole-cell recording was performed with pipette solutions in which the ability to buffer changes in intracellular calcium was varied by altering the concentration of the calcium chelator (2-aminophenoxy)-ethane-N,N,N',N'-tetraacetic acid (BAPTA). Flufenamic acid (2 x 10(-4) M) and niflumic acid (5 x 10(-4) M) both evoked large outward currents when recordings were made with either 1 x 10(-4) M or 1 x 10(-2) M BAPTA. Furthermore, bathing the cells in nominally calcium-free extracellular solution did not reduce the amplitude of the evoked currents. 9. It is concluded that both flufenamic and mefenamic acid inhibit ICl(Ca) by a mechanism similar to niflumic acid, possibly open channel blockade. Furthermore, at concentrations greater than 5 x 10(-5) M all three fenamates inhibited STOC activity and evoked directly an outward current which resembled IK(Ca).
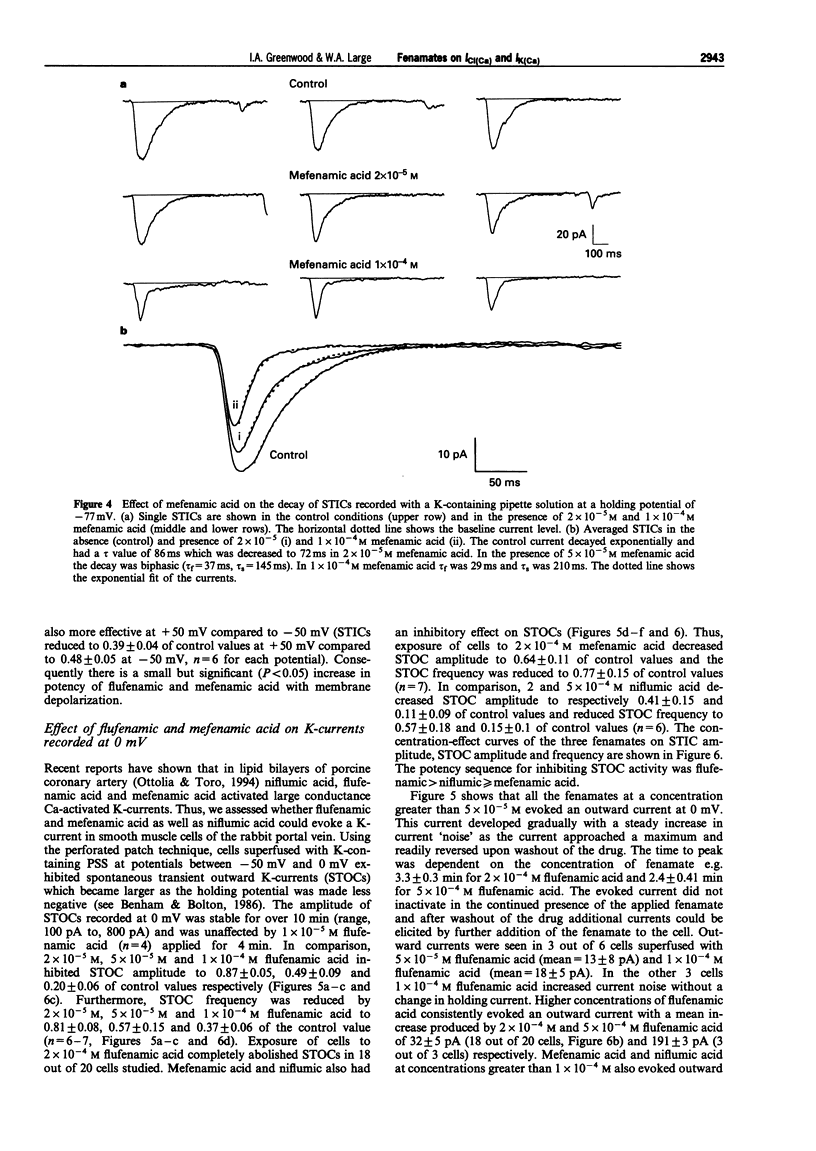
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akbarali H. I., Giles W. R. Ca2+ and Ca(2+)-activated Cl- currents in rabbit oesophageal smooth muscle. J Physiol. 1993 Jan;460:117–133. doi: 10.1113/jphysiol.1993.sp019462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beech D. J., Bolton T. B. Two components of potassium current activated by depolarization of single smooth muscle cells from the rabbit portal vein. J Physiol. 1989 Nov;418:293–309. doi: 10.1113/jphysiol.1989.sp017841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beech D. J., Zhang H., Nakao K., Bolton T. B. Single channel and whole-cell K-currents evoked by levcromakalim in smooth muscle cells from the rabbit portal vein. Br J Pharmacol. 1993 Oct;110(2):583–590. doi: 10.1111/j.1476-5381.1993.tb13850.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benham C. D., Bolton T. B. Spontaneous transient outward currents in single visceral and vascular smooth muscle cells of the rabbit. J Physiol. 1986 Dec;381:385–406. doi: 10.1113/jphysiol.1986.sp016333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards G., Niederste-Hollenberg A., Schneider J., Noack T., Weston A. H. Ion channel modulation by NS 1619, the putative BKCa channel opener, in vascular smooth muscle. Br J Pharmacol. 1994 Dec;113(4):1538–1547. doi: 10.1111/j.1476-5381.1994.tb17171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogg R. C., Wang Q., Large W. A. Action of niflumic acid on evoked and spontaneous calcium-activated chloride and potassium currents in smooth muscle cells from rabbit portal vein. Br J Pharmacol. 1994 Jul;112(3):977–984. doi: 10.1111/j.1476-5381.1994.tb13177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogg R. C., Wang Q., Large W. A. Effects of Cl channel blockers on Ca-activated chloride and potassium currents in smooth muscle cells from rabbit portal vein. Br J Pharmacol. 1994 Apr;111(4):1333–1341. doi: 10.1111/j.1476-5381.1994.tb14891.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogg R. C., Wang Q., Large W. A. Time course of spontaneous calcium-activated chloride currents in smooth muscle cells from the rabbit portal vein. J Physiol. 1993 May;464:15–31. doi: 10.1113/jphysiol.1993.sp019622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen L. J., Sims S. M. Acetylcholine activates non-selective cation and chloride conductances in canine and guinea-pig tracheal myocytes. J Physiol. 1992;453:197–218. doi: 10.1113/jphysiol.1992.sp019224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb F. S., Volk K. A., Shibata E. F. Calcium-activated chloride current in rabbit coronary artery myocytes. Circ Res. 1994 Oct;75(4):742–750. doi: 10.1161/01.res.75.4.742. [DOI] [PubMed] [Google Scholar]
- McManus O. B., Harris G. H., Giangiacomo K. M., Feigenbaum P., Reuben J. P., Addy M. E., Burka J. F., Kaczorowski G. J., Garcia M. L. An activator of calcium-dependent potassium channels isolated from a medicinal herb. Biochemistry. 1993 Jun 22;32(24):6128–6133. doi: 10.1021/bi00075a002. [DOI] [PubMed] [Google Scholar]
- Noack T., Deitmer P., Edwards G., Weston A. H. Characterization of potassium currents modulated by BRL 38227 in rat portal vein. Br J Pharmacol. 1992 Jul;106(3):717–726. doi: 10.1111/j.1476-5381.1992.tb14400.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottolia M., Toro L. Potentiation of large conductance KCa channels by niflumic, flufenamic, and mefenamic acids. Biophys J. 1994 Dec;67(6):2272–2279. doi: 10.1016/S0006-3495(94)80712-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacaud P., Loirand G., Lavie J. L., Mironneau C., Mironneau J. Calcium-activated chloride current in rat vascular smooth muscle cells in short-term primary culture. Pflugers Arch. 1989 Apr;413(6):629–636. doi: 10.1007/BF00581813. [DOI] [PubMed] [Google Scholar]
- Uyama Y., Imaizumi Y., Watanabe M. Cyclopiazonic acid, an inhibitor of Ca(2+)-ATPase in sarcoplasmic reticulum, increases excitability in ileal smooth muscle. Br J Pharmacol. 1993 Oct;110(2):565–572. doi: 10.1111/j.1476-5381.1993.tb13848.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q., Hogg R. C., Large W. A. Properties of spontaneous inward currents recorded in smooth muscle cells isolated from the rabbit portal vein. J Physiol. 1992;451:525–537. doi: 10.1113/jphysiol.1992.sp019177. [DOI] [PMC free article] [PubMed] [Google Scholar]


