Abstract
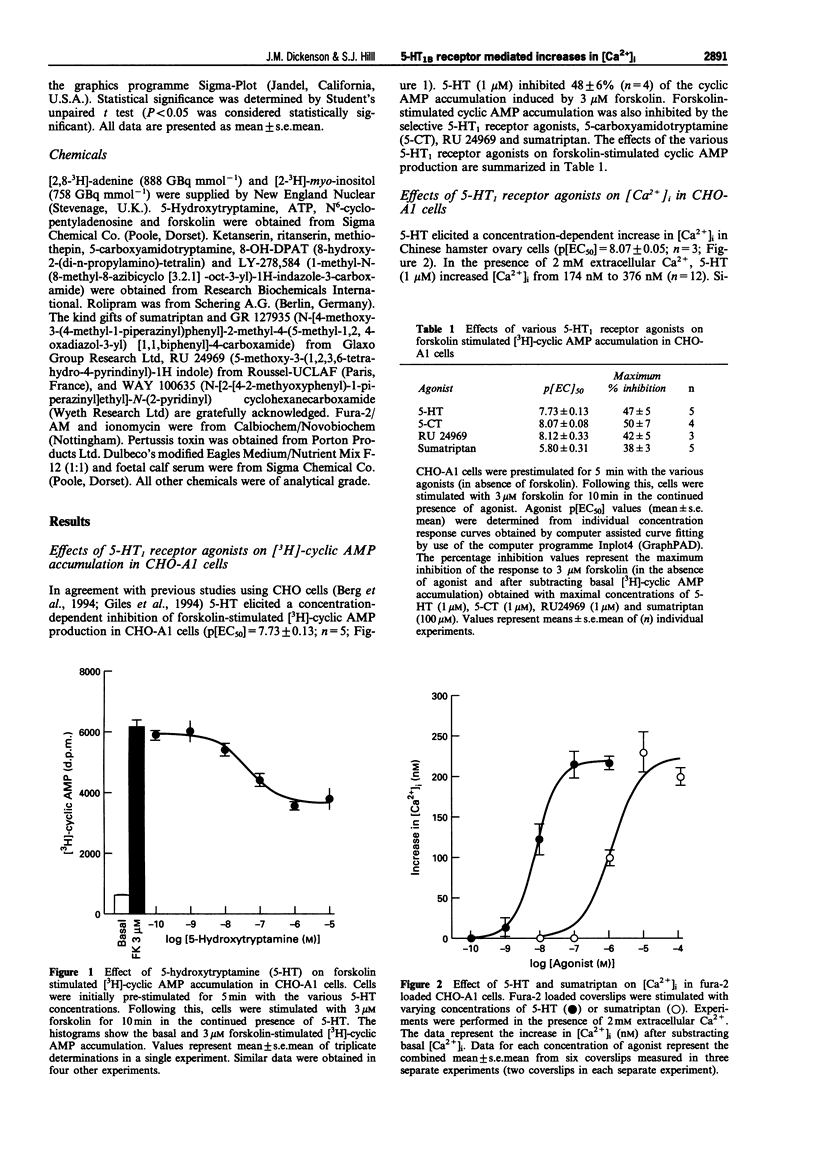
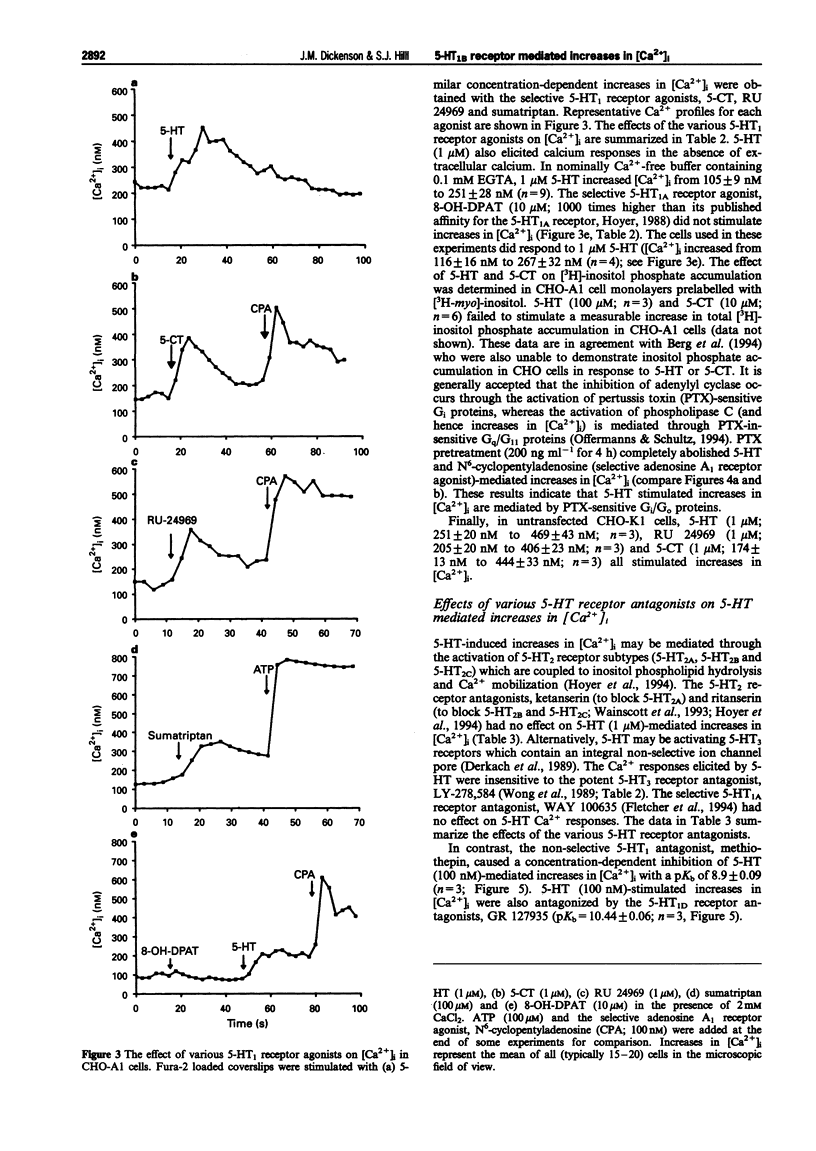
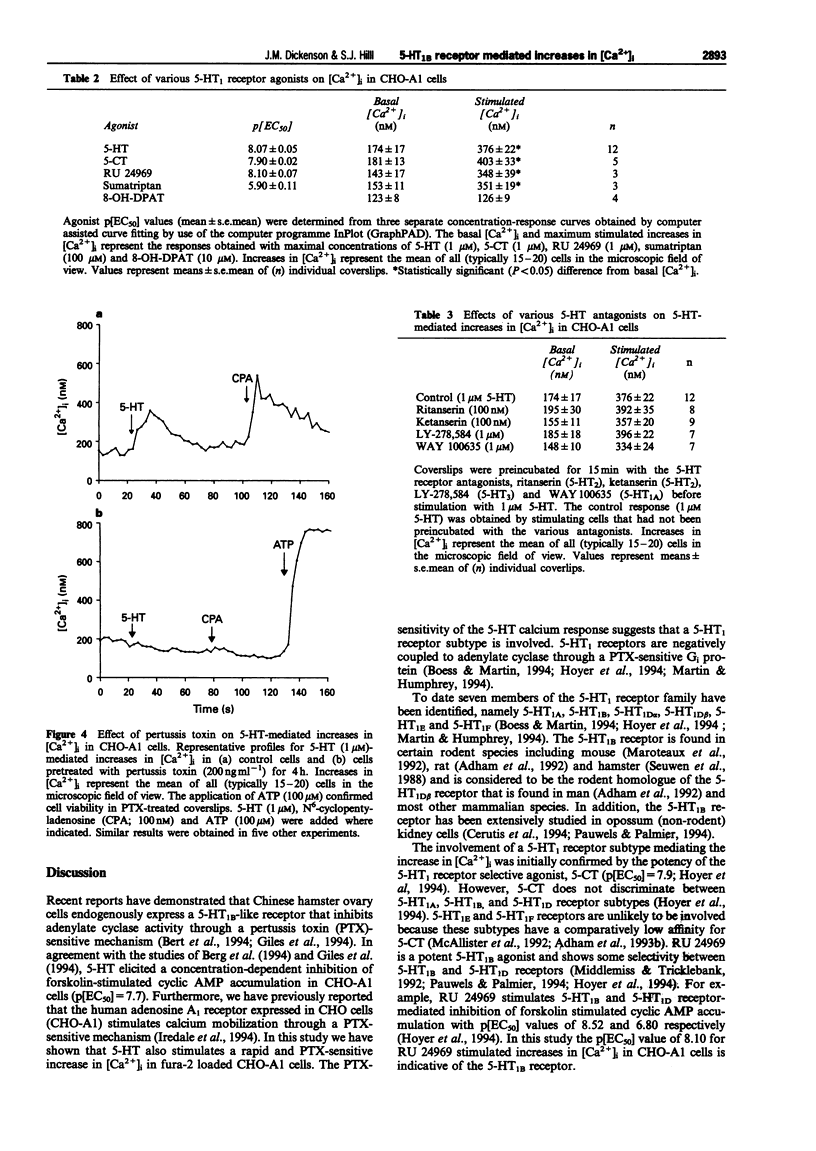
1. Chinese hamster ovary cells (CHO-K1) express an endogenous 5-hydroxytryptamine (5-HT)1B-like receptor that is negatively coupled to adenylyl cyclase through a pertussis toxin (PTX)-sensitive mechanism. Furthermore, the human adenosine A1 receptor when expressed in CHO-K1 cells (CHO-A1) has been shown to mobilize intracellular Ca2+ through a PTX-sensitive mechanism. Therefore the aim of this investigation was to determine whether the endogenous 5-HT1B-like receptor was able to stimulate increases in intracellular free [Ca2+] ([Ca2+]i) in CHO-A1 cells. 2. In agreement with previous studies using CHO cells, 5-hydroxytryptamine (5-HT) elicited a concentration-dependent inhibition of forskolin-stimulated [3H]-cyclic AMP production in CHO-A1 cells (p[EC50] = 7.73 +/- 0.13). 5-HT (1 microM) inhibited 47 +/- 5% of the [3H]-cyclic AMP accumulation induced by 3 microM forskolin. Forskolin stimulated [3H]-cyclic AMP accumulation was also inhibited by the 5-HT1 receptor agonists (p[EC50] values) 5-carboxyamidotryptamine (5-CT; 8.07 +/- 0.08), RU 24969 (8.12 +/- 0.33) and sumatriptan (5.80 +/- 0.31). 3. 5-HT elicited a concentration-dependent increase in [Ca2+]i in CHO-A1 cells (p[EC50] = 8.07 +/- 0.05). In the presence of 2 mM extracellular Ca2+, 5-HT (1 microM) increased [Ca2+]i from 174 +/- 17 nM to 376 +/- 22 nM. The 5-HT1 receptor agonists (p[EC50] values), 5-carboxyamidotryptamine (5-CT; 7.9 +/- 0.02), RU 24969 (8.1 +/- 0.07) and sumatriptan (5.9 +/- 0.11) all elicited concentration-dependent increases in [Ca2+]i. Similar maximal increases in [Ca2+]i were obtained with each agonist. The selective 5-HT1A receptor agonist, 8-OH-DPAT (10 microM) did not stimulate increases in [Ca2+]i. 5-HT (100 microM) and 5-CT (10 microM) did not stimulate a measurable increase in [3H]-inositol phosphate accumulation in CHO-A1 cells. 4. 5-HT (1 microM)-mediated increases in [Ca2+]i were insensitive to the 5-HT receptor antagonist, ritanserin (5-HT2; 100 nM), ketanserin (5-HT2; 100 nM), LY-278,584 (5-HT3; 1 microM) and WAY 100635 (5-HT1A; 1 microM). The response to 5-HT (100 nM) was antagonized by the non-selective 5-HT1 antagonist, methiothepin (pKb = 8.90 +/- 0.09) and the 5-HT1D antagonist GR 127935 (pKb = 10.44 +/- 0.06). 5. Pretreatment with PTX (200 ng ml-1 for 4 h) completely attenuated the Ca2+ response to 100 microM 5-HT. 6. In untransfected CHO-K1 cells, 5-HT (1 microM), RU 24969 (1 microM), and 5-CT (1 microM) elicited increases in [Ca2+]i similar to those observed in CHO-A1 cells. 7. These data demonstrate that in CHO-K1 cells the endogenously expressed 5-HT1B-like receptor couples to the phospholipase C/Ca2+ signalling pathway through a PTX-sensitive pathway, suggesting the involvement of Gi/Go protein(s).
Full text
PDF

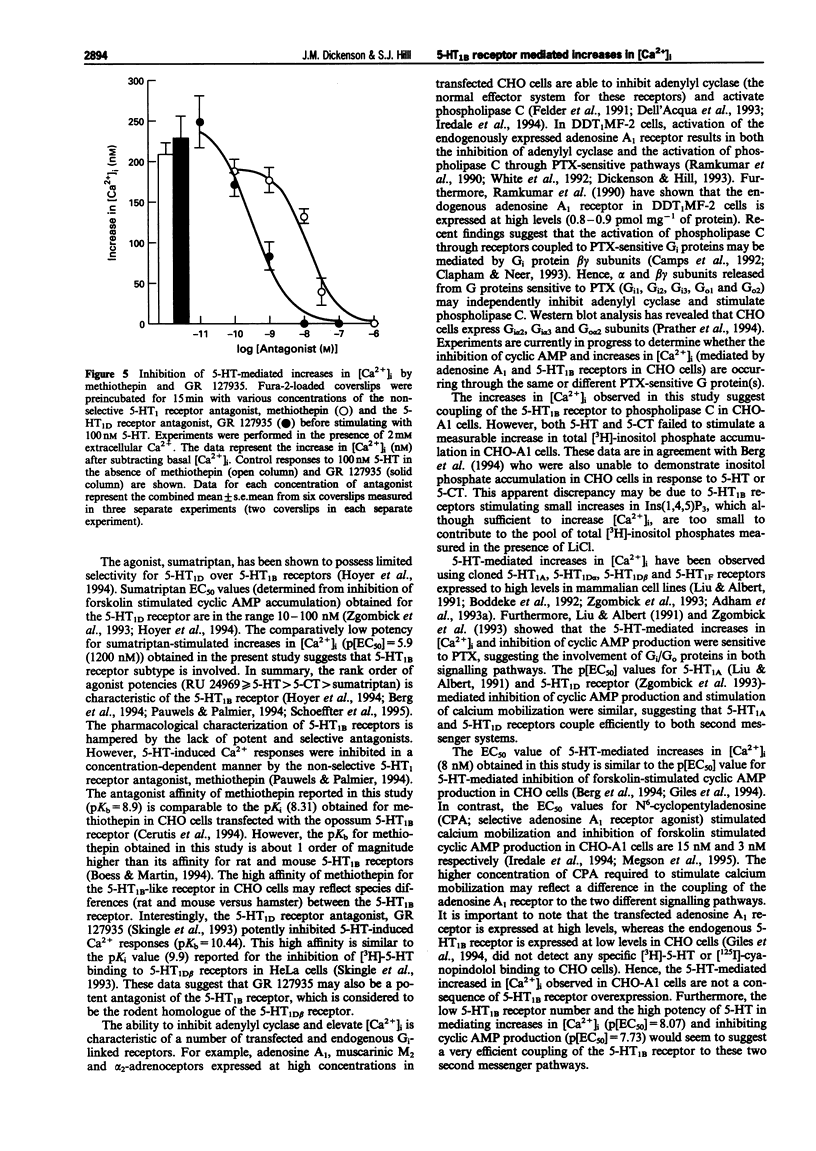


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adham N., Kao H. T., Schecter L. E., Bard J., Olsen M., Urquhart D., Durkin M., Hartig P. R., Weinshank R. L., Branchek T. A. Cloning of another human serotonin receptor (5-HT1F): a fifth 5-HT1 receptor subtype coupled to the inhibition of adenylate cyclase. Proc Natl Acad Sci U S A. 1993 Jan 15;90(2):408–412. doi: 10.1073/pnas.90.2.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adham N., Romanienko P., Hartig P., Weinshank R. L., Branchek T. The rat 5-hydroxytryptamine1B receptor is the species homologue of the human 5-hydroxytryptamine1D beta receptor. Mol Pharmacol. 1992 Jan;41(1):1–7. [PubMed] [Google Scholar]
- Berg K. A., Clarke W. P., Sailstad C., Saltzman A., Maayani S. Signal transduction differences between 5-hydroxytryptamine type 2A and type 2C receptor systems. Mol Pharmacol. 1994 Sep;46(3):477–484. [PubMed] [Google Scholar]
- Boddeke H. W., Fargin A., Raymond J. R., Schoeffter P., Hoyer D. Agonist/antagonist interactions with cloned human 5-HT1A receptors: variations in intrinsic activity studied in transfected HeLa cells. Naunyn Schmiedebergs Arch Pharmacol. 1992 Mar;345(3):257–263. doi: 10.1007/BF00168684. [DOI] [PubMed] [Google Scholar]
- Boess F. G., Martin I. L. Molecular biology of 5-HT receptors. Neuropharmacology. 1994 Mar-Apr;33(3-4):275–317. doi: 10.1016/0028-3908(94)90059-0. [DOI] [PubMed] [Google Scholar]
- Camps M., Carozzi A., Schnabel P., Scheer A., Parker P. J., Gierschik P. Isozyme-selective stimulation of phospholipase C-beta 2 by G protein beta gamma-subunits. Nature. 1992 Dec 17;360(6405):684–686. doi: 10.1038/360684a0. [DOI] [PubMed] [Google Scholar]
- Cerutis D. R., Hass N. A., Iversen L. J., Bylund D. B. The cloning and expression of an OK cell cDNA encoding a 5-hydroxytryptamine1B receptor. Mol Pharmacol. 1994 Jan;45(1):20–28. [PubMed] [Google Scholar]
- Choppin A., O'Connor S. E. Presence of vasoconstrictor 5HT1-like receptors revealed by precontraction of rabbit isolated mesenteric artery. Br J Pharmacol. 1995 Jan;114(2):309–314. doi: 10.1111/j.1476-5381.1995.tb13228.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapham D. E., Neer E. J. New roles for G-protein beta gamma-dimers in transmembrane signalling. Nature. 1993 Sep 30;365(6445):403–406. doi: 10.1038/365403a0. [DOI] [PubMed] [Google Scholar]
- Dell'Acqua M. L., Carroll R. C., Peralta E. G. Transfected m2 muscarinic acetylcholine receptors couple to G alpha i2 and G alpha i3 in Chinese hamster ovary cells. Activation and desensitization of the phospholipase C signaling pathway. J Biol Chem. 1993 Mar 15;268(8):5676–5685. [PubMed] [Google Scholar]
- Derkach V., Surprenant A., North R. A. 5-HT3 receptors are membrane ion channels. Nature. 1989 Jun 29;339(6227):706–709. doi: 10.1038/339706a0. [DOI] [PubMed] [Google Scholar]
- Dickenson J. M., Hill S. J. Adenosine A1-receptor stimulated increases in intracellular calcium in the smooth muscle cell line, DDT1MF-2. Br J Pharmacol. 1993 Jan;108(1):85–92. doi: 10.1111/j.1476-5381.1993.tb13444.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson J., Brown A. M., Hill S. J. Influence of rolipram on the cyclic 3',5'-adenosine monophosphate response to histamine and adenosine in slices of guinea-pig cerebral cortex. Biochem Pharmacol. 1988 Feb 15;37(4):715–723. doi: 10.1016/0006-2952(88)90146-3. [DOI] [PubMed] [Google Scholar]
- Fargin A., Raymond J. R., Regan J. W., Cotecchia S., Lefkowitz R. J., Caron M. G. Effector coupling mechanisms of the cloned 5-HT1A receptor. J Biol Chem. 1989 Sep 5;264(25):14848–14852. [PubMed] [Google Scholar]
- Felder C. C., Williams H. L., Axelrod J. A transduction pathway associated with receptors coupled to the inhibitory guanine nucleotide binding protein Gi that amplifies ATP-mediated arachidonic acid release. Proc Natl Acad Sci U S A. 1991 Aug 1;88(15):6477–6480. doi: 10.1073/pnas.88.15.6477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hoyer D., Clarke D. E., Fozard J. R., Hartig P. R., Martin G. R., Mylecharane E. J., Saxena P. R., Humphrey P. P. International Union of Pharmacology classification of receptors for 5-hydroxytryptamine (Serotonin). Pharmacol Rev. 1994 Jun;46(2):157–203. [PubMed] [Google Scholar]
- Hoyer D. Functional correlates of serotonin 5-HT1 recognition sites. J Recept Res. 1988;8(1-4):59–81. doi: 10.3109/10799898809048978. [DOI] [PubMed] [Google Scholar]
- Iredale P. A., Alexander S. P., Hill S. J. Coupling of a transfected human brain A1 adenosine receptor in CHO-K1 cells to calcium mobilisation via a pertussis toxin-sensitive mechanism. Br J Pharmacol. 1994 Apr;111(4):1252–1256. doi: 10.1111/j.1476-5381.1994.tb14880.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y. F., Albert P. R. Cell-specific signaling of the 5-HT1A receptor. Modulation by protein kinases C and A. J Biol Chem. 1991 Dec 15;266(35):23689–23697. [PubMed] [Google Scholar]
- Maroteaux L., Saudou F., Amlaiky N., Boschert U., Plassat J. L., Hen R. Mouse 5HT1B serotonin receptor: cloning, functional expression, and localization in motor control centers. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):3020–3024. doi: 10.1073/pnas.89.7.3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin G. R., Humphrey P. P. Receptors for 5-hydroxytryptamine: current perspectives on classification and nomenclature. Neuropharmacology. 1994 Mar-Apr;33(3-4):261–273. doi: 10.1016/0028-3908(94)90058-2. [DOI] [PubMed] [Google Scholar]
- McAllister G., Charlesworth A., Snodin C., Beer M. S., Noble A. J., Middlemiss D. N., Iversen L. L., Whiting P. Molecular cloning of a serotonin receptor from human brain (5HT1E): a fifth 5HT1-like subtype. Proc Natl Acad Sci U S A. 1992 Jun 15;89(12):5517–5521. doi: 10.1073/pnas.89.12.5517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Megson A. C., Dickenson J. M., Townsend-Nicholson A., Hill S. J. Synergy between the inositol phosphate responses to transfected human adenosine A1-receptors and constitutive P2-purinoceptors in CHO-K1 cells. Br J Pharmacol. 1995 Aug;115(8):1415–1424. doi: 10.1111/j.1476-5381.1995.tb16632.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middlemiss D. N., Tricklebank M. D. Centrally active 5-HT receptor agonists and antagonists. Neurosci Biobehav Rev. 1992 Spring;16(1):75–82. doi: 10.1016/s0149-7634(05)80054-5. [DOI] [PubMed] [Google Scholar]
- Milligan G. Mechanisms of multifunctional signalling by G protein-linked receptors. Trends Pharmacol Sci. 1993 Jun;14(6):239–244. doi: 10.1016/0165-6147(93)90019-g. [DOI] [PubMed] [Google Scholar]
- Offermanns S., Schultz G. Complex information processing by the transmembrane signaling system involving G proteins. Naunyn Schmiedebergs Arch Pharmacol. 1994 Oct;350(4):329–338. doi: 10.1007/BF00178947. [DOI] [PubMed] [Google Scholar]
- Parsons A. A., Stutchbury C., Raval P., Kaumann A. J. Sumatriptan contracts large coronary arteries of beagle dogs through 5-HT1-like receptors. Naunyn Schmiedebergs Arch Pharmacol. 1992 Nov;346(5):592–596. doi: 10.1007/BF00169018. [DOI] [PubMed] [Google Scholar]
- Pauwels P. J., Palmier C. Inhibition by 5-HT of forskolin-induced cAMP formation in the renal opossum epithelial cell line OK: mediation by a 5-HT1B like receptor and antagonism by methiothepin. Neuropharmacology. 1994 Jan;33(1):67–75. doi: 10.1016/0028-3908(94)90098-1. [DOI] [PubMed] [Google Scholar]
- Prather P. L., McGinn T. M., Erickson L. J., Evans C. J., Loh H. H., Law P. Y. Ability of delta-opioid receptors to interact with multiple G-proteins is independent of receptor density. J Biol Chem. 1994 Aug 19;269(33):21293–21302. [PubMed] [Google Scholar]
- Ramkumar V., Barrington W. W., Jacobson K. A., Stiles G. L. Demonstration of both A1 and A2 adenosine receptors in DDT1 MF-2 smooth muscle cells. Mol Pharmacol. 1990 Feb;37(2):149–156. [PMC free article] [PubMed] [Google Scholar]
- Ruck A., Millns P., Kendall D. A., Hill S. J. Expression of beta 2-adrenoceptors mediating cyclic AMP accumulation in astroglial and neuronal cell lines derived from the rat CNS. Biochem Pharmacol. 1990 Nov 15;40(10):2371–2375. doi: 10.1016/0006-2952(90)90735-4. [DOI] [PubMed] [Google Scholar]
- Schoeffter P., Pfeilschifter J., Bobirnac I. 5-Hydroxytryptamine 5-HT1B receptors inhibiting cyclic AMP accumulation in rat renal mesangial cells. Naunyn Schmiedebergs Arch Pharmacol. 1995 Jan;351(1):35–39. doi: 10.1007/BF00169061. [DOI] [PubMed] [Google Scholar]
- Seuwen K., Magnaldo I., Pouysségur J. Serotonin stimulates DNA synthesis in fibroblasts acting through 5-HT1B receptors coupled to a Gi-protein. Nature. 1988 Sep 15;335(6187):254–256. doi: 10.1038/335254a0. [DOI] [PubMed] [Google Scholar]
- Stiles G. L. Adenosine receptors. J Biol Chem. 1992 Apr 5;267(10):6451–6454. [PubMed] [Google Scholar]
- Townsend-Nicholson A., Shine J. Molecular cloning and characterisation of a human brain A1 adenosine receptor cDNA. Brain Res Mol Brain Res. 1992 Dec;16(3-4):365–370. doi: 10.1016/0169-328x(92)90248-a. [DOI] [PubMed] [Google Scholar]
- Wainscott D. B., Cohen M. L., Schenck K. W., Audia J. E., Nissen J. S., Baez M., Kursar J. D., Lucaites V. L., Nelson D. L. Pharmacological characteristics of the newly cloned rat 5-hydroxytryptamine2F receptor. Mol Pharmacol. 1993 Mar;43(3):419–426. [PubMed] [Google Scholar]
- White T. E., Dickenson J. M., Alexander S. P., Hill S. J. Adenosine A1-receptor stimulation of inositol phospholipid hydrolysis and calcium mobilisation in DDT1 MF-2 cells. Br J Pharmacol. 1992 May;106(1):215–221. doi: 10.1111/j.1476-5381.1992.tb14317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong D. T., Robertson D. W., Reid L. R. Specific [3H]LY278584 binding to 5-HT3 recognition sites in rat cerebral cortex. Eur J Pharmacol. 1989 Jul 4;166(1):107–110. doi: 10.1016/0014-2999(89)90689-4. [DOI] [PubMed] [Google Scholar]
- Zgombick J. M., Borden L. A., Cochran T. L., Kucharewicz S. A., Weinshank R. L., Branchek T. A. Dual coupling of cloned human 5-hydroxytryptamine1D alpha and 5-hydroxytryptamine1D beta receptors stably expressed in murine fibroblasts: inhibition of adenylate cyclase and elevation of intracellular calcium concentrations via pertussis toxin-sensitive G protein(s). Mol Pharmacol. 1993 Sep;44(3):575–582. [PubMed] [Google Scholar]


