Abstract
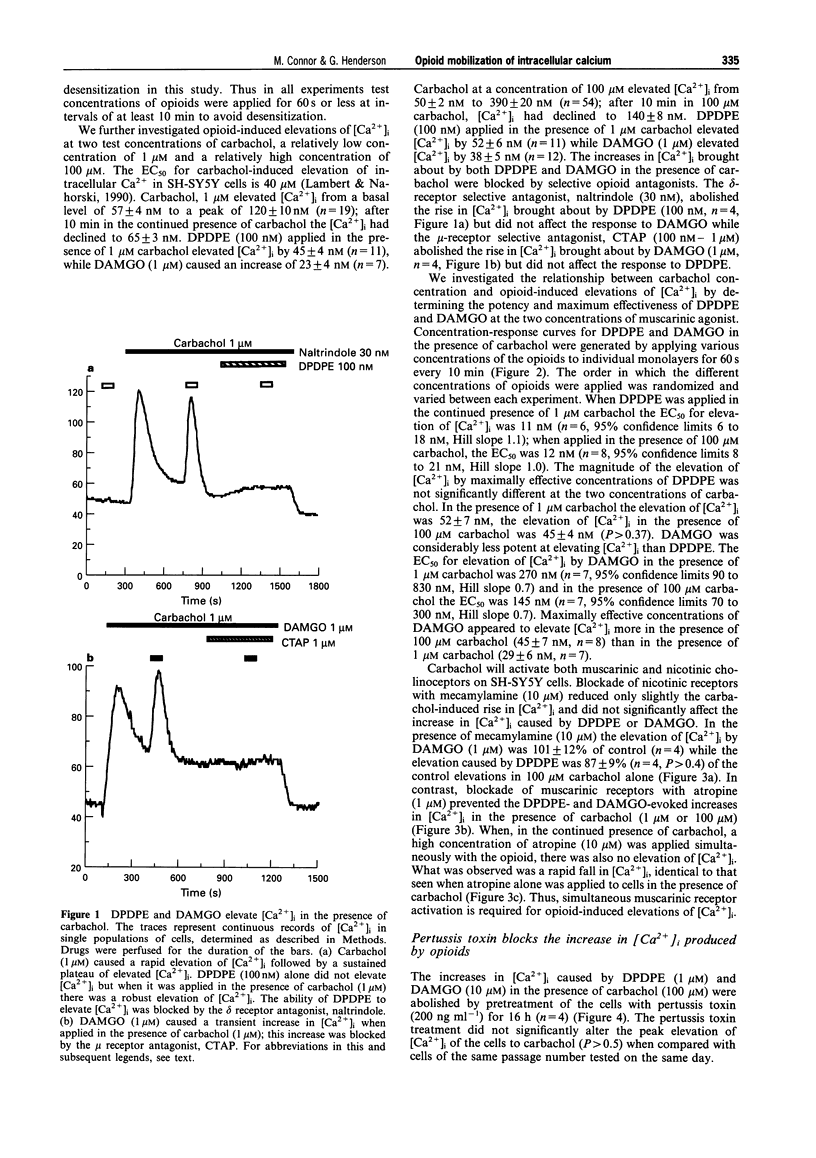
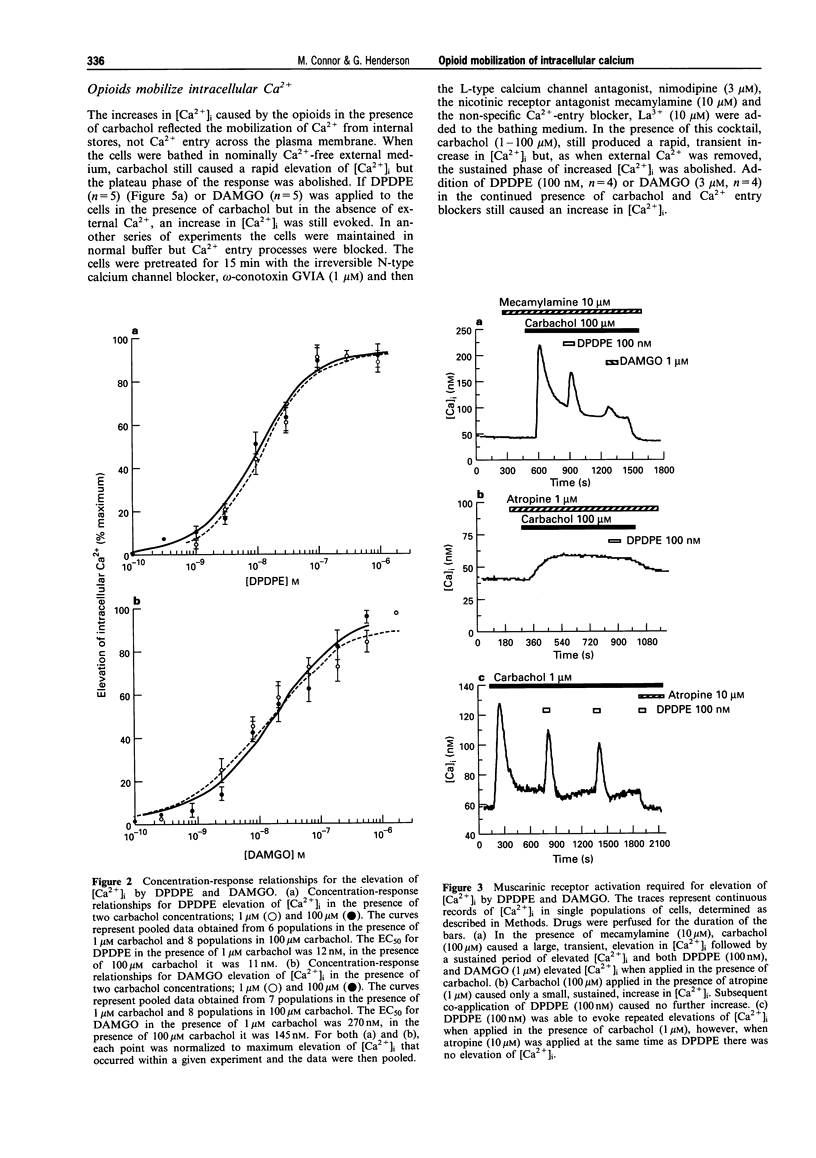
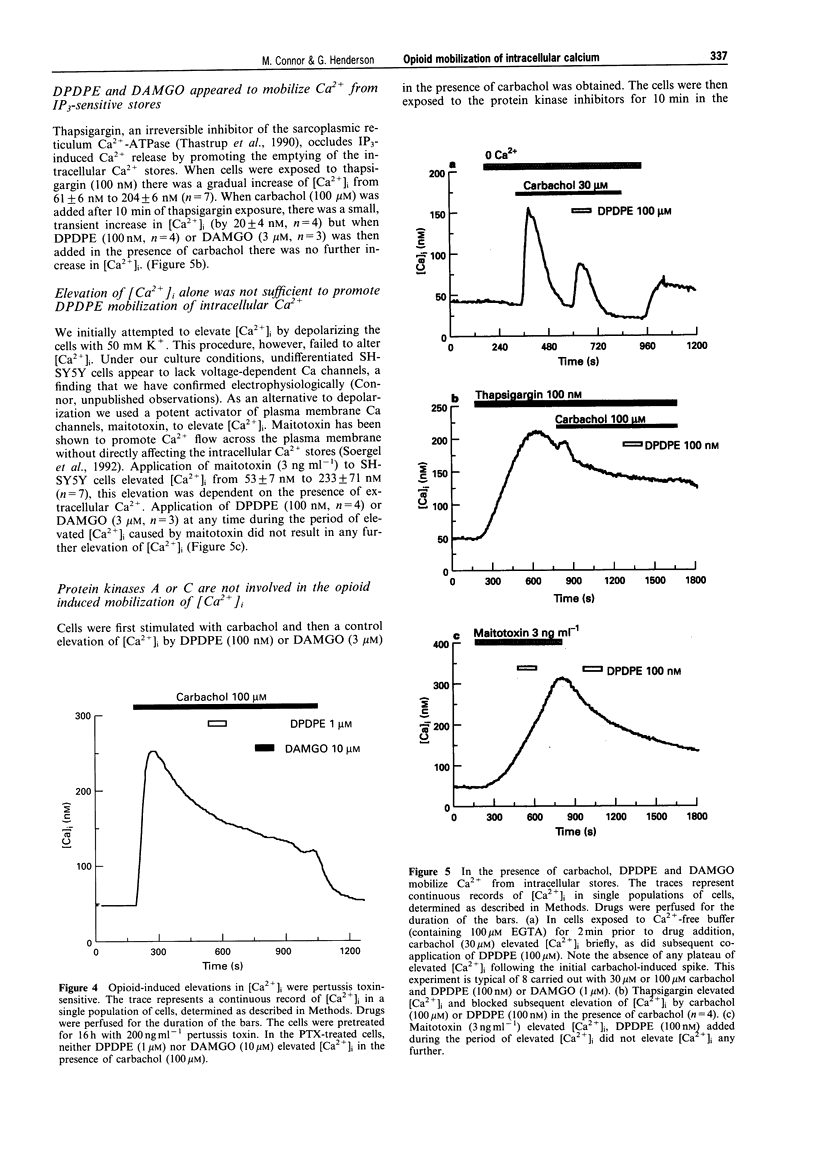
1. In this study we have investigated delta and mu opioid receptor-mediated elevation of intracellular Ca2+ concentration ([Ca2+]i) in the human neuroblastoma cell line, SH-SY5Y. 2. The Ca(2+)-sensitive dye, fura-2, was used to measure [Ca2+]i in confluent monolayers of SH-SY5Y cells. Neither the delta-opioid agonist, DPDPE ([D-Pen2,5]-enkephalin) nor the mu-opioid agonist, DAMGO (Tyr-D-Ala-Gly-N-Me-Phe-Gly-ol enkephalin) elevated [Ca2+]i when applied alone. However, when either DPDPE or DAMGO was applied in the presence of the cholinoceptor agonist, carbachol (100 nM-1 mM) they evoked an elevation of [Ca2+]i above that caused by carbachol alone. 3. In the presence of 1 microM or 100 microM carbachol, DPDPE elevated [Ca2+]i with an EC50 of 10 nM. The elevation of [Ca2+]i was independent of the concentration of carbachol. The EC50 for DAMGO elevating [Ca2+]i in the presence of 1 microM and 100 microM carbachol was 270 nM and 145 nM respectively. 4. The delta-receptor antagonist, naltrindole (30 nM), blocked the elevations of [Ca2+]i by DPDPE (100 nM) without affecting those caused by DAMGO while the mu-receptor antagonist, CTAP (D-Phe-Cys-Tyr-D-Trp-Arg-Pen-Thr-NH2) (100 nM-1 microM) blocked the elevations of [Ca2+]i caused by DAMGO (1 microM) without affecting those caused by DPDPE. 5. Block of carbachol activation of muscarinic receptors with atropine (10 microM) abolished the elevation of [Ca2+]i by the opioids. The nicotinic receptor antagonist, mecamylamine (10 microM), did not affect the elevations of [Ca2+]i caused by opioids in the presence of carbachol. 6. Muscarinic receptor activation, not a rise in [Ca2+]i, was required to reveal the opioid response. The Ca2+ channel activator, maitotoxin (3 ng ml-1), also elevated [Ca2+]i but subsequent application of opioid in the presence of maitotoxin caused no further changes in [Ca2+]i. 7. The elevations of [Ca2+]i by DPDPE and DAMGO were abolished by pretreatment of the cells with pertussis toxin (200 ng ml-1, 16 h). This treatment did not significantly affect the response of the cells to carbachol. 8. The opioids appeared to elevate [Ca2+]i by mobilizing Ca2+ from intracellular stores. Both DPDPE and DAMGO continued to elevate [Ca2+]i when applied in nominally Ca(2+)-free external buffer or when applied in a buffer containing a cocktail of Ca2+ entry inhibitors. Thapsigargin (100 nM), an agent which discharges intracellular Ca2+ stores, also blocked the opioid elevations of [Ca2+]i. 9. delta and mu Opioids did not appear to mobilize intracellular Ca2+ by modulating the activity of protein kinases. The application of H-89 (10 microM), an inhibitor of protein kinase A, H-7 (100 microM), an inhibitor of protein kinase C, protein kinase A and cyclic GMP-dependent protein kinase, or Bis I, an inhibitor of protein kinase C, did not alter the opioid mobilization of [Ca2+]i. 10. Thus, in SH-SY5Y cells, opioids can mobilize Ca2+ from intracellular stores but they require ongoing muscarinic receptor activation. Opioids do not elevate [Ca2+]i when applied alone.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chen L., Huang L. Y. Sustained potentiation of NMDA receptor-mediated glutamate responses through activation of protein kinase C by a mu opioid. Neuron. 1991 Aug;7(2):319–326. doi: 10.1016/0896-6273(91)90270-a. [DOI] [PubMed] [Google Scholar]
- Chijiwa T., Mishima A., Hagiwara M., Sano M., Hayashi K., Inoue T., Naito K., Toshioka T., Hidaka H. Inhibition of forskolin-induced neurite outgrowth and protein phosphorylation by a newly synthesized selective inhibitor of cyclic AMP-dependent protein kinase, N-[2-(p-bromocinnamylamino)ethyl]-5-isoquinolinesulfonamide (H-89), of PC12D pheochromocytoma cells. J Biol Chem. 1990 Mar 25;265(9):5267–5272. [PubMed] [Google Scholar]
- Collier H. O., Roy A. C. Morphine-like drugs inhibit the stimulation of E prostaglandins of cyclic AMP formation by rat brain homogenate. Nature. 1974 Mar 1;248(5443):24–27. doi: 10.1038/248024a0. [DOI] [PubMed] [Google Scholar]
- Eriksson P. S., Nilsson M., Wågberg M., Hansson E., Rönnbäck L. Kappa-opioid receptors on astrocytes stimulate L-type Ca2+ channels. Neuroscience. 1993 May;54(2):401–407. doi: 10.1016/0306-4522(93)90261-d. [DOI] [PubMed] [Google Scholar]
- Exton J. H. Phosphoinositide phospholipases and G proteins in hormone action. Annu Rev Physiol. 1994;56:349–369. doi: 10.1146/annurev.ph.56.030194.002025. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Gucker S., Bidlack J. M. Protein kinase C activation increases the rate and magnitude of agonist-induced delta-opioid receptor down-regulation in NG108-15 cells. Mol Pharmacol. 1992 Oct;42(4):656–665. [PubMed] [Google Scholar]
- Hidaka H., Inagaki M., Kawamoto S., Sasaki Y. Isoquinolinesulfonamides, novel and potent inhibitors of cyclic nucleotide dependent protein kinase and protein kinase C. Biochemistry. 1984 Oct 9;23(21):5036–5041. doi: 10.1021/bi00316a032. [DOI] [PubMed] [Google Scholar]
- Jain S., Maheshwari M. C., Dhamija R. M., Mishra N. K. Pure motor hemiparesis due to hypertensive putaminal haemorrhage. Eur Neurol. 1985;24(3):205–207. doi: 10.1159/000115795. [DOI] [PubMed] [Google Scholar]
- Jin W., Lee N. M., Loh H. H., Thayer S. A. Dual excitatory and inhibitory effects of opioids on intracellular calcium in neuroblastoma x glioma hybrid NG108-15 cells. Mol Pharmacol. 1992 Dec;42(6):1083–1089. [PubMed] [Google Scholar]
- Jin W., Lee N. M., Loh H. H., Thayer S. A. Opioids mobilize calcium from inositol 1,4,5-trisphosphate-sensitive stores in NG108-15 cells. J Neurosci. 1994 Apr;14(4):1920–1929. doi: 10.1523/JNEUROSCI.14-04-01920.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazmi S. M., Mishra R. K. Comparative pharmacological properties and functional coupling of mu and delta opioid receptor sites in human neuroblastoma SH-SY5Y cells. Mol Pharmacol. 1987 Jul;32(1):109–118. [PubMed] [Google Scholar]
- Kazmi S. M., Mishra R. K. Opioid receptors in human neuroblastoma SH-SY5Y cells: evidence for distinct morphine (mu) and enkephalin (delta) binding sites. Biochem Biophys Res Commun. 1986 Jun 13;137(2):813–820. doi: 10.1016/0006-291x(86)91152-6. [DOI] [PubMed] [Google Scholar]
- Lambert D. G., Nahorski S. R. Muscarinic-receptor-mediated changes in intracellular Ca2+ and inositol 1,4,5-trisphosphate mass in a human neuroblastoma cell line, SH-SY5Y. Biochem J. 1990 Jan 15;265(2):555–562. doi: 10.1042/bj2650555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laugwitz K. L., Offermanns S., Spicher K., Schultz G. mu and delta opioid receptors differentially couple to G protein subtypes in membranes of human neuroblastoma SH-SY5Y cells. Neuron. 1993 Feb;10(2):233–242. doi: 10.1016/0896-6273(93)90314-h. [DOI] [PubMed] [Google Scholar]
- Okajima F., Kondo Y. Synergism in cytosolic Ca2+ mobilization between bradykinin and agonists for pertussis toxin-sensitive G-protein-coupled receptors in NG 108-15 cells. FEBS Lett. 1992 Apr 20;301(2):223–226. doi: 10.1016/0014-5793(92)81252-h. [DOI] [PubMed] [Google Scholar]
- Okajima F., Tomura H., Kondo Y. Enkephalin activates the phospholipase C/Ca2+ system through cross-talk between opioid receptors and P2-purinergic or bradykinin receptors in NG 108-15 cells. A permissive role for pertussis toxin-sensitive G-proteins. Biochem J. 1993 Feb 15;290(Pt 1):241–247. doi: 10.1042/bj2900241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olianas M. C., Onali P. Synergistic interaction of muscarinic and opioid receptors with GS-linked neurotransmitter receptors to stimulate adenylyl cyclase activity of rat olfactory bulb. J Neurochem. 1993 Dec;61(6):2183–2190. doi: 10.1111/j.1471-4159.1993.tb07458.x. [DOI] [PubMed] [Google Scholar]
- Pickles R. J., Cuthbert A. W. Relation of anion secretory activity to intracellular Ca2+ in response to lysylbradykinin and histamine in a cultured human colonic epithelium. Eur J Pharmacol. 1991 Jun 18;199(1):77–91. doi: 10.1016/0014-2999(91)90639-8. [DOI] [PubMed] [Google Scholar]
- Prather P. L., McGinn T. M., Erickson L. J., Evans C. J., Loh H. H., Law P. Y. Ability of delta-opioid receptors to interact with multiple G-proteins is independent of receptor density. J Biol Chem. 1994 Aug 19;269(33):21293–21302. [PubMed] [Google Scholar]
- Prather P. L., Tsai A. W., Law P. Y. Mu and delta opioid receptor desensitization in undifferentiated human neuroblastoma SHSY5Y cells. J Pharmacol Exp Ther. 1994 Jul;270(1):177–184. [PubMed] [Google Scholar]
- Seward E., Hammond C., Henderson G. Mu-opioid-receptor-mediated inhibition of the N-type calcium-channel current. Proc Biol Sci. 1991 May 22;244(1310):129–135. doi: 10.1098/rspb.1991.0061. [DOI] [PubMed] [Google Scholar]
- Simonin F., Befort K., Gavériaux-Ruff C., Matthes H., Nappey V., Lannes B., Micheletti G., Kieffer B. The human delta-opioid receptor: genomic organization, cDNA cloning, functional expression, and distribution in human brain. Mol Pharmacol. 1994 Dec;46(6):1015–1021. [PubMed] [Google Scholar]
- Smart D., Smith G., Lambert D. G. Mu-opioids activate phospholipase C in SH-SY5Y human neuroblastoma cells via calcium-channel opening. Biochem J. 1995 Jan 15;305(Pt 2):577–581. doi: 10.1042/bj3050577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart D., Smith G., Lambert D. G. mu-Opioid receptor stimulation of inositol (1,4,5)trisphosphate formation via a pertussis toxin-sensitive G protein. J Neurochem. 1994 Mar;62(3):1009–1014. doi: 10.1046/j.1471-4159.1994.62031009.x. [DOI] [PubMed] [Google Scholar]
- Soergel D. G., Yasumoto T., Daly J. W., Gusovsky F. Maitotoxin effects are blocked by SK&F 96365, an inhibitor of receptor-mediated calcium entry. Mol Pharmacol. 1992 Mar;41(3):487–493. [PubMed] [Google Scholar]
- Supattapone S., Danoff S. K., Theibert A., Joseph S. K., Steiner J., Snyder S. H. Cyclic AMP-dependent phosphorylation of a brain inositol trisphosphate receptor decreases its release of calcium. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8747–8750. doi: 10.1073/pnas.85.22.8747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang T., Kiang J. G., Cox B. M. Opioids acting through delta receptors elicit a transient increase in the intracellular free calcium concentration in dorsal root ganglion-neuroblastoma hybrid ND8-47 cells. J Pharmacol Exp Ther. 1994 Jul;270(1):40–46. [PubMed] [Google Scholar]
- Tang W. J., Gilman A. G. Type-specific regulation of adenylyl cyclase by G protein beta gamma subunits. Science. 1991 Dec 6;254(5037):1500–1503. doi: 10.1126/science.1962211. [DOI] [PubMed] [Google Scholar]
- Thastrup O., Cullen P. J., Drøbak B. K., Hanley M. R., Dawson A. P. Thapsigargin, a tumor promoter, discharges intracellular Ca2+ stores by specific inhibition of the endoplasmic reticulum Ca2(+)-ATPase. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2466–2470. doi: 10.1073/pnas.87.7.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomura H., Okajima F., Kondo Y. Enkephalin induces Ca2+ mobilization in single cells of bradykinin-sensitized differentiated neuroblastoma hybridoma (NG108-15) cells. Neurosci Lett. 1992 Dec 14;148(1-2):93–96. doi: 10.1016/0304-3940(92)90812-l. [DOI] [PubMed] [Google Scholar]
- Toullec D., Pianetti P., Coste H., Bellevergue P., Grand-Perret T., Ajakane M., Baudet V., Boissin P., Boursier E., Loriolle F. The bisindolylmaleimide GF 109203X is a potent and selective inhibitor of protein kinase C. J Biol Chem. 1991 Aug 25;266(24):15771–15781. [PubMed] [Google Scholar]
- Wojcikiewicz R. J., Tobin A. B., Nahorski S. R. Desensitization of cell signalling mediated by phosphoinositidase C. Trends Pharmacol Sci. 1993 Jul;14(7):279–285. doi: 10.1016/0165-6147(93)90131-3. [DOI] [PubMed] [Google Scholar]
- Yu V. C., Sadée W. Efficacy and tolerance of narcotic analgesics at the mu opioid receptor in differentiated human neuroblastoma cells. J Pharmacol Exp Ther. 1988 Apr;245(1):350–355. [PubMed] [Google Scholar]



