Abstract
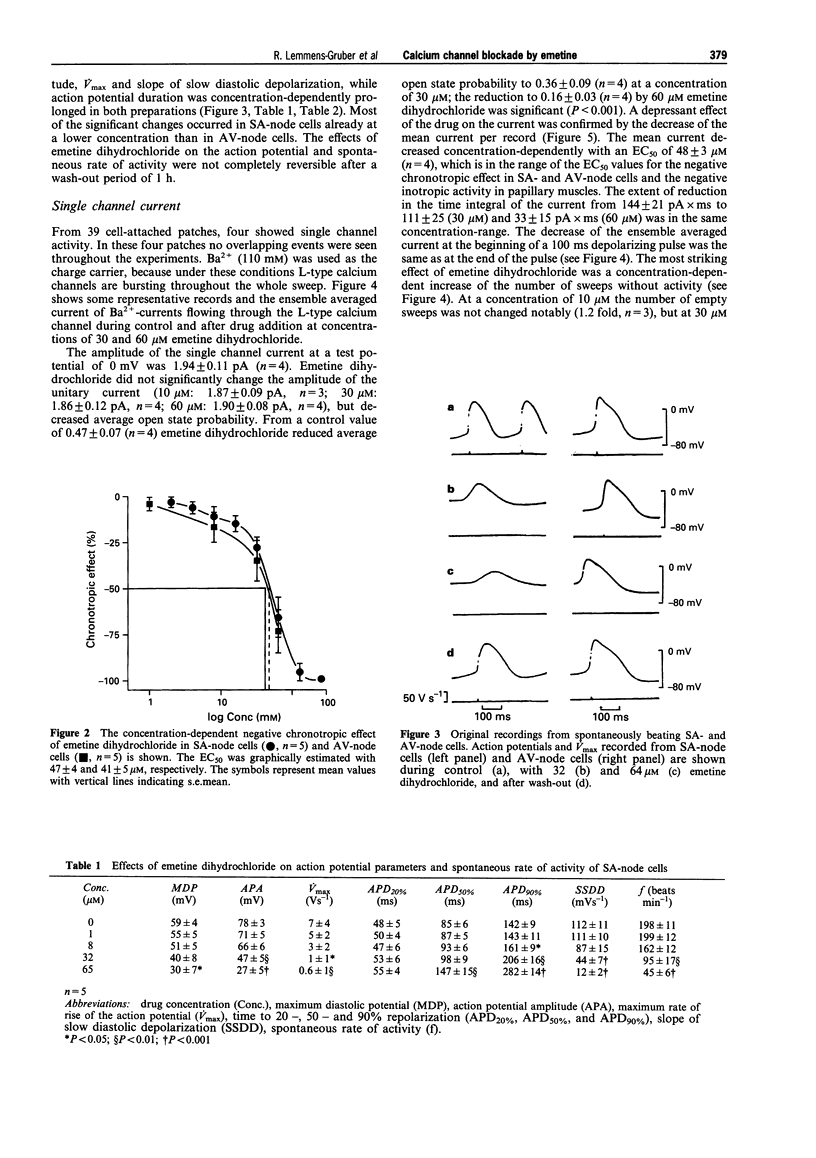
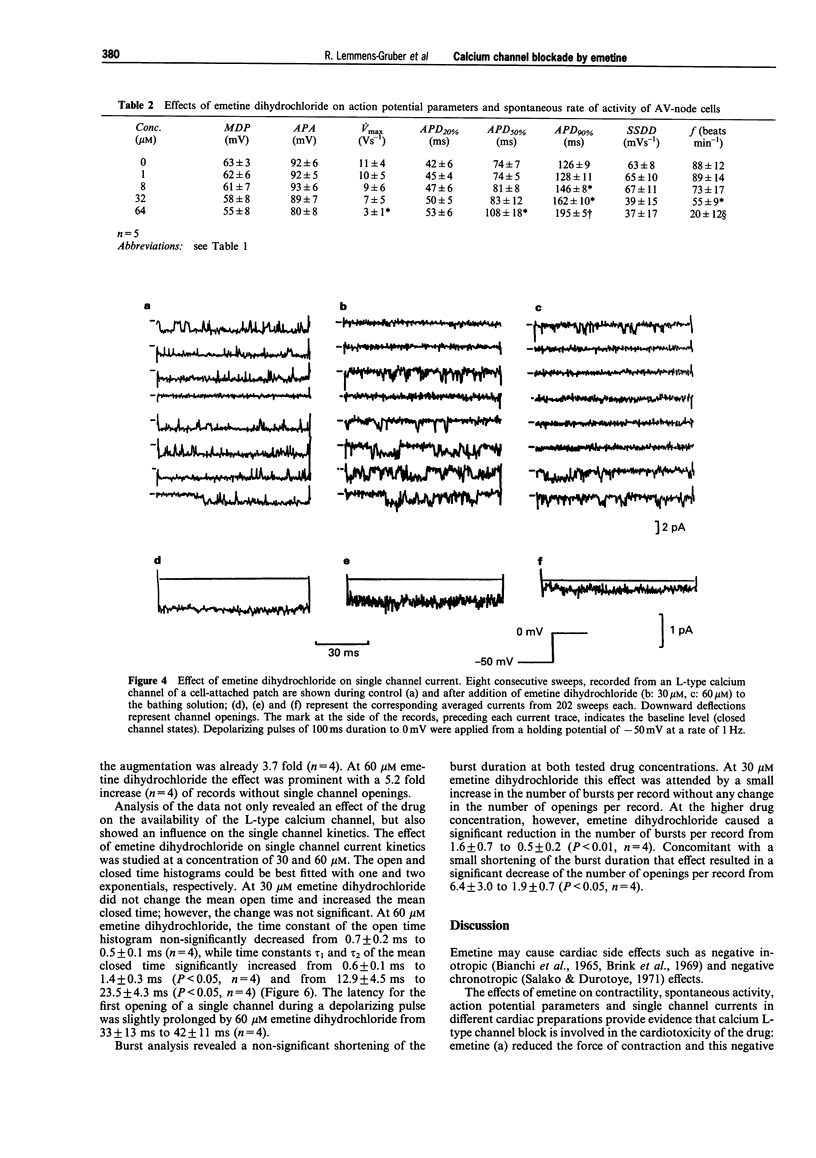
1. The cardiotoxic effects of emetine dihydrochloride on mechanical and electrical activity were studied in isolated preparations (papillary muscles, sinoatrial and atrioventricular nodes, ventricular myocytes) of the guinea-pig heart. 2. Force of contraction was measured isometrically, action potentials and maximum rate of rise of the action potential were recorded by means of the intracellular microelectrode technique. Single channel L-type calcium current (Ba2+ ions as charge carrier) was studied with the patch-clamp technique in the cell-attached mode. 3. Emetine dihydrochloride (8-256 microM) reduced force of contraction in papillary muscles and spontaneous activity of sinoatrial and atrioventricular nodes concentration-dependently; the negative inotropic effect was abolished when the extracellular Ca2+ concentration was increased. 4. Maximum diastolic potential, action potential amplitude, maximum rate of rise of the action potential and the slope of the slow diastolic depolarization were decreased by emetine in sinoatrial as well as atrioventricular noes, while action potential duration was prolonged in both preparations (1-64 microM). 5. The amplitude of the L-type calcium single channel current was not altered by emetine dihydrochloride, while average open state probability was decreased concentration-dependently (10, 30 and 60 microM). 6. The most prominent effect of emetine dihydrochloride on single channel current was an increase of sweeps without activity. 7. At 60 microM, emetine dihydrochloride caused a decrease of the mean open time an increase of the mean closed time. The number of openings per record and number of bursts per record were reduced. 8. It is concluded that emetine dihydrochloride produces an L-type calcium channel block which might contribute to its cardiac side effects.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler A. G., Walinsky P., Krall R. A., Cho S. Y. Death resulting from ipecac syrup poisoning. JAMA. 1980 May 16;243(19):1927–1928. [PubMed] [Google Scholar]
- Bayer R., Hennekes R., Kaufmann R., Mannhold R. Inotropic and electrophysiological actions of verapamil and D 600 in mammalian myocardium. I. Pattern of inotropic effects of the racemic compounds. Naunyn Schmiedebergs Arch Pharmacol. 1975;290(1):49–68. doi: 10.1007/BF00499989. [DOI] [PubMed] [Google Scholar]
- Bayer R., Rodenkirchen R., Kaufmann R., Lee J. H., Hennekes R. The effects of nifedipine on contraction and monophasic action potential of isolated cat myocardium. Naunyn Schmiedebergs Arch Pharmacol. 1977 Dec;301(1):29–37. doi: 10.1007/BF00501261. [DOI] [PubMed] [Google Scholar]
- Beeler G. W., Reuter H. Reconstruction of the action potential of ventricular myocardial fibres. J Physiol. 1977 Jun;268(1):177–210. doi: 10.1113/jphysiol.1977.sp011853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi A., De Marino V., De Vleeschhouwer G. R., Marino A. Effects of emetine on heart, coronary circulation and blood pressure (research in vitro on the guinea pig heart and coronary flow; research in vivo on the dog heart, coronary flow and blood pressure). Arch Int Pharmacodyn Ther. 1965 Jul;156(1):238–248. [PubMed] [Google Scholar]
- Brink A. J., Kotzé J. C., Muller S. P., Lochner A. The effect of emetine on metabolism and contractility of the isolated rat heart. J Pharmacol Exp Ther. 1969 Feb;165(2):251–257. [PubMed] [Google Scholar]
- Combs A. B., Acosta D. Toxic mechanisms of the heart: a review. Toxicol Pathol. 1990;18(4 Pt 1):583–596. [PubMed] [Google Scholar]
- Cranefield P. F., Aronson R. S., Wit A. L. Effect of verapamil on the noraml action potential and on a calcium-dependent slow response of canine cardiac Purkinje fibers. Circ Res. 1974 Feb;34(2):204–213. doi: 10.1161/01.res.34.2.204. [DOI] [PubMed] [Google Scholar]
- Dresser L. P., Massey E. W., Johnson E. E., Bossen E. Ipecac myopathy and cardiomyopathy. J Neurol Neurosurg Psychiatry. 1993 May;56(5):560–562. doi: 10.1136/jnnp.56.5.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Droogmans G., Nilius B. Kinetic properties of the cardiac T-type calcium channel in the guinea-pig. J Physiol. 1989 Dec;419:627–650. doi: 10.1113/jphysiol.1989.sp017890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenwick E. M., Marty A., Neher E. Sodium and calcium channels in bovine chromaffin cells. J Physiol. 1982 Oct;331:599–635. doi: 10.1113/jphysiol.1982.sp014394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleckenstein A. Specific pharmacology of calcium in myocardium, cardiac pacemakers, and vascular smooth muscle. Annu Rev Pharmacol Toxicol. 1977;17:149–166. doi: 10.1146/annurev.pa.17.040177.001053. [DOI] [PubMed] [Google Scholar]
- Friedman E. J. Death from ipecac intoxication in a patient with anorexia nervosa. Am J Psychiatry. 1984 May;141(5):702–703. doi: 10.1176/ajp.141.5.702. [DOI] [PubMed] [Google Scholar]
- Hachisu M., Pappano A. J. A comparative study of the blockade of calcium-dependent action potentials by verapamil, nifedipine and nimodipine in ventricular muscle. J Pharmacol Exp Ther. 1983 Apr;225(1):112–120. [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hess P., Lansman J. B., Tsien R. W. Different modes of Ca channel gating behaviour favoured by dihydropyridine Ca agonists and antagonists. Nature. 1984 Oct 11;311(5986):538–544. doi: 10.1038/311538a0. [DOI] [PubMed] [Google Scholar]
- Kass R. S. Delayed rectification in the cardiac Purkinje fiber is not activated by intracellular calcium. Biophys J. 1984 Apr;45(4):837–839. doi: 10.1016/S0006-3495(84)84227-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kass R. S. Nisoldipine: a new, more selective calcium current blocker in cardiac Purkinje fibers. J Pharmacol Exp Ther. 1982 Nov;223(2):446–456. [PubMed] [Google Scholar]
- Kawai C., Konishi T., Matsuyama E., Okazaki H. Comparative effects of three calcium antagonists, diltiazem, verapamil and nifedipine, on the sinoatrial and atrioventricular nodes. Experimental and clinical studies. Circulation. 1981 May;63(5):1035–1042. doi: 10.1161/01.cir.63.5.1035. [DOI] [PubMed] [Google Scholar]
- Kawashima Y., Ochi R. Voltage-dependent decrease in the availability of single calcium channels by nitrendipine in guinea-pig ventricular cells. J Physiol. 1988 Aug;402:219–235. doi: 10.1113/jphysiol.1988.sp017201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohlhardt M. Saturation characteristics, Ca2+ ions and drug-induced block of cardiac Isi-mediated action potentials. Naunyn Schmiedebergs Arch Pharmacol. 1983 Jul;323(3):251–260. doi: 10.1007/BF00497671. [DOI] [PubMed] [Google Scholar]
- Kuntzer T., Bogousslavsky J., Deruaz J. P., Janzer R., Regli F. Reversible emetine-induced myopathy with ECG abnormalities: a toxic myopathy. J Neurol. 1989 May;236(4):246–248. doi: 10.1007/BF00314508. [DOI] [PubMed] [Google Scholar]
- Lee K. S., Tsien R. W. Mechanism of calcium channel blockade by verapamil, D600, diltiazem and nitrendipine in single dialysed heart cells. Nature. 1983 Apr 28;302(5911):790–794. doi: 10.1038/302790a0. [DOI] [PubMed] [Google Scholar]
- Marino A., Costa R., De Natale G. La cardiotossicità dell'emetina. Clin Ter. 1990 May 15;133(3):131–143. [PubMed] [Google Scholar]
- Mateer J. E., Farrell B. J., Chou S. S., Gutmann L. Reversible ipecac myopathy. Arch Neurol. 1985 Feb;42(2):188–190. doi: 10.1001/archneur.1985.04060020106024. [DOI] [PubMed] [Google Scholar]
- Mitra R., Morad M. A uniform enzymatic method for dissociation of myocytes from hearts and stomachs of vertebrates. Am J Physiol. 1985 Nov;249(5 Pt 2):H1056–H1060. doi: 10.1152/ajpheart.1985.249.5.H1056. [DOI] [PubMed] [Google Scholar]
- Nakajima H., Hoshiyama M., Yamashita K., Kiyomoto A. Effect of diltiazem on electrical and mechanical activity of isolated cardiac ventricular muscle of guinea pig. Jpn J Pharmacol. 1975 Aug;25(4):383–392. doi: 10.1254/jjp.25.383. [DOI] [PubMed] [Google Scholar]
- Näbauer M., Callewaert G., Cleemann L., Morad M. Regulation of calcium release is gated by calcium current, not gating charge, in cardiac myocytes. Science. 1989 May 19;244(4906):800–803. doi: 10.1126/science.2543067. [DOI] [PubMed] [Google Scholar]
- Palmer E. P., Guay A. T. Reversible myopathy secondary to abuse of ipecac in patients with major eating disorders. N Engl J Med. 1985 Dec 5;313(23):1457–1459. doi: 10.1056/NEJM198512053132306. [DOI] [PubMed] [Google Scholar]
- Reiter M. Die Wertbestimmung inotrop wirkender Arzneimittel am isolierten Papillarmuskel. Arzneimittelforschung. 1967 Oct;17(10):1249–1253. [PubMed] [Google Scholar]
- Salako L. A., Durotoye A. O. Observations on the hypotensive response to dehydrometine. Eur J Pharmacol. 1971 Apr;14(2):200–203. doi: 10.1016/0014-2999(71)90212-3. [DOI] [PubMed] [Google Scholar]
- Schiff R. J., Wurzel C. L., Brunson S. C., Kasloff I., Nussbaum M. P., Frank S. D. Death due to chronic syrup of ipecac use in a patient with bulimia. Pediatrics. 1986 Sep;78(3):412–416. [PubMed] [Google Scholar]
- Stuiver P. C., Visser L. G. Het amoeboom van de dikke darm en het rectum. Ned Tijdschr Geneeskd. 1993 Nov 6;137(45):2328–2331. [PubMed] [Google Scholar]
- Thyagarajan D., Day B. J., Wodak J., Gilligan B., Dennett X. Emetine myopathy in a patient with an eating disorder. Med J Aust. 1993 Dec 6;159(11-12):757–760. doi: 10.5694/j.1326-5377.1993.tb141340.x. [DOI] [PubMed] [Google Scholar]
- Tohse N. Calcium-sensitive delayed rectifier potassium current in guinea pig ventricular cells. Am J Physiol. 1990 Apr;258(4 Pt 2):H1200–H1207. doi: 10.1152/ajpheart.1990.258.4.H1200. [DOI] [PubMed] [Google Scholar]



