Abstract
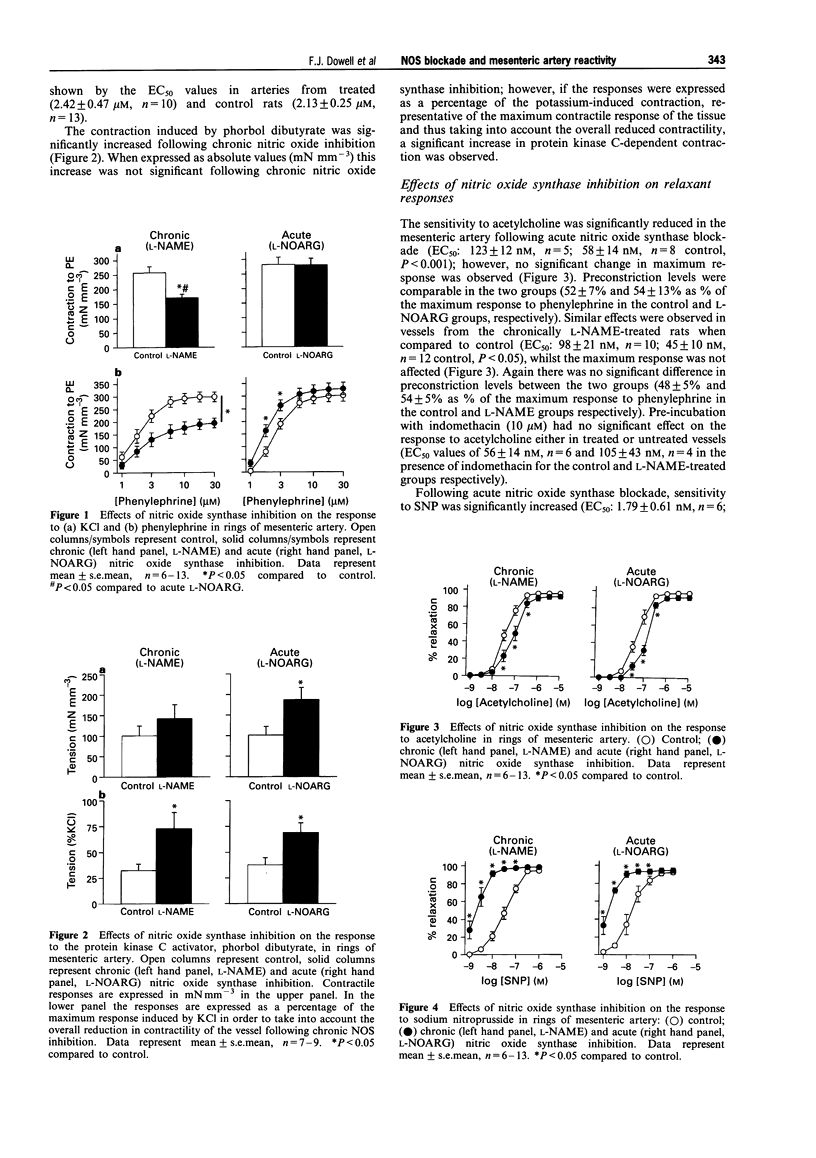
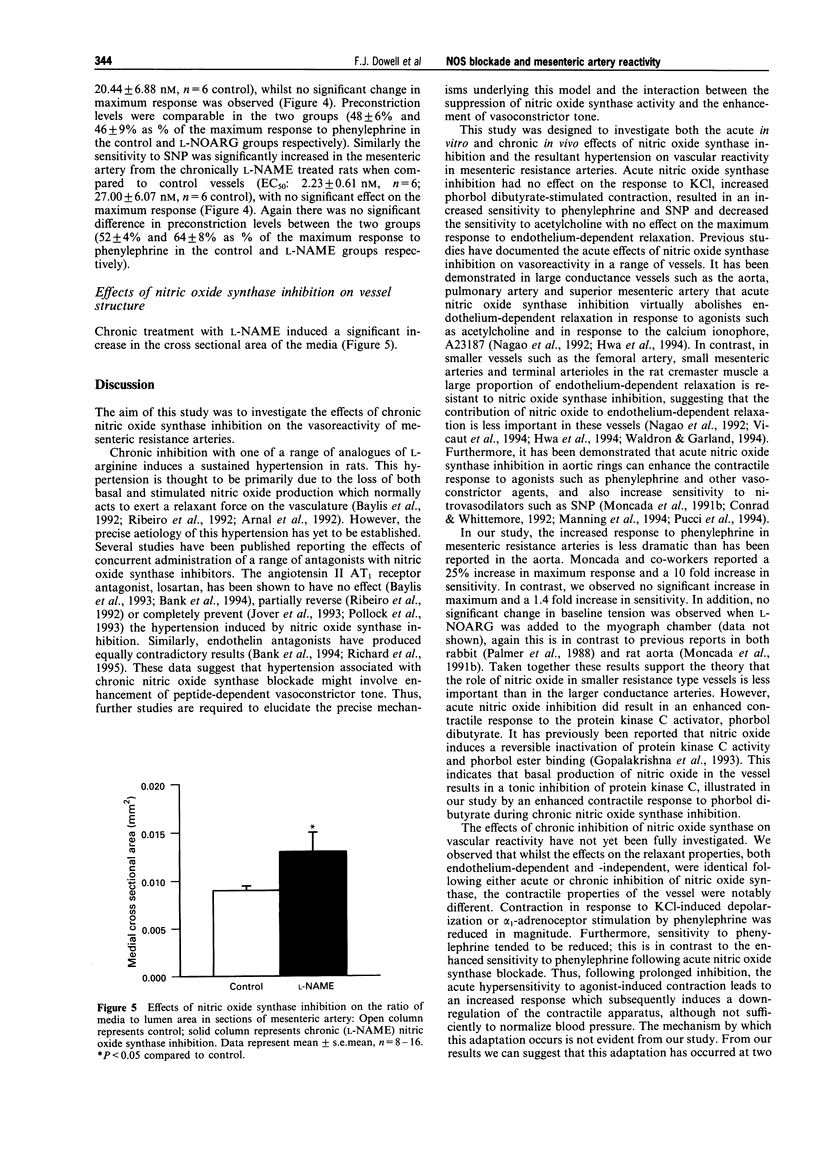
1. Chronic inhibition of nitric oxide synthase (NOS) induces a sustained hypertension in rats. We studied the effects of chronic inhibition on the in vitro vasoreactivity of mesenteric resistance arteries in Wistar rats. We also investigated the effects of acute in vitro NOS inhibition in these vessels. 2. Acute NOS inhibition (N omega-nitro-L-arginine, L-NOARG, 10 microM) had no effect on the contractile response to KCl (125 mM), enhanced the response to the phorbol ester, phorbol dibutyrate (1 microM; 69 +/- 9% of KCl response, n = 6; 38 +/- 7% control, n = 6, P < 0.05), increased sensitivity to phenylephrine (EC50: 1.68 +/- 0.14 microM, n = 5; 2.35 +/- 0.23 microM control, n = 5, P < 0.05) and sodium nitroprusside (SNP; EC50 1.79 +/- 0.61 nM, n = 6; 20.44 +/- 6.87 nM control, n = 6, P < 0.05) and decreased sensitivity to acetylcholine (EC50 123 +/- 12 nM, n = 6; 45 +/- 10 nM control, n = 13, P < 0.05). 3. In contrast, contractile responses to KCl (125 mM; 170 +/- 12 mN mm-3, n = 10; 257 +/- 21 mN mm-3 in control, n = 13, P < 0.005) and phenylephrine (maximum response, 30 microM: 169 +/- 24 mN mm-3, n = 10; 295 +/- 19 mN mm-3 in control, n = 13, P < 0.001) were significantly reduced in magnitude following chronic NOS inhibition. Sensitivity to phenylephrine was not significantly altered. 4. The effects of chronic NOS inhibition (N omega-nitro-L-arginine methyl ester, L-NAME, 10 mg kg-1 daily for 3 weeks) were similar to those of acute NOS blockade with respect to the relaxant responses to SNP and acetylcholine, and also the contraction in response to protein kinase C activation. 5. Chronic inhibition of NOS significantly increased medial cross sectional area of mesenteric resistance arteries (0.013 +/- 0.002 mm2, n = 7; 0.009 +/- 0.0005 mm2 control, n = 15, P < 0.05). 6. Thus, in contrast to the acute effects of NOS inhibition, chronic NOS inhibition results in a down-regulation of the contractile responses to KCl and phenylephrine in mesenteric resistance arteries, despite an increase in medial cross sectional area. However protein kinase C-dependent contraction remains relatively enhanced. Endothelium-dependent relaxation is reduced and endothelium-independent relaxation is enhanced in a manner similar to the effects of acute NOS blockade.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnal J. F., Warin L., Michel J. B. Determinants of aortic cyclic guanosine monophosphate in hypertension induced by chronic inhibition of nitric oxide synthase. J Clin Invest. 1992 Aug;90(2):647–652. doi: 10.1172/JCI115906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnal J. F., el Amrani A. I., Chatellier G., Ménard J., Michel J. B. Cardiac weight in hypertension induced by nitric oxide synthase blockade. Hypertension. 1993 Sep;22(3):380–387. doi: 10.1161/01.hyp.22.3.380. [DOI] [PubMed] [Google Scholar]
- Bank N., Aynedjian H. S., Khan G. A. Mechanism of vasoconstriction induced by chronic inhibition of nitric oxide in rats. Hypertension. 1994 Sep;24(3):322–328. doi: 10.1161/01.hyp.24.3.322. [DOI] [PubMed] [Google Scholar]
- Baylis C., Engels K., Samsell L., Harton P. Renal effects of acute endothelial-derived relaxing factor blockade are not mediated by angiotensin II. Am J Physiol. 1993 Jan;264(1 Pt 2):F74–F78. doi: 10.1152/ajprenal.1993.264.1.F74. [DOI] [PubMed] [Google Scholar]
- Baylis C., Mitruka B., Deng A. Chronic blockade of nitric oxide synthesis in the rat produces systemic hypertension and glomerular damage. J Clin Invest. 1992 Jul;90(1):278–281. doi: 10.1172/JCI115849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen K. L., Mulvany M. J. Mesenteric arcade arteries contribute substantially to vascular resistance in conscious rats. J Vasc Res. 1993 Mar-Apr;30(2):73–79. doi: 10.1159/000158978. [DOI] [PubMed] [Google Scholar]
- Conrad K. P., Whittemore S. L. NG-monomethyl-L-arginine and nitroarginine potentiate pressor responsiveness of vasoconstrictors in conscious rats. Am J Physiol. 1992 Jun;262(6 Pt 2):R1137–R1144. doi: 10.1152/ajpregu.1992.262.6.R1137. [DOI] [PubMed] [Google Scholar]
- Cornwell T. L., Arnold E., Boerth N. J., Lincoln T. M. Inhibition of smooth muscle cell growth by nitric oxide and activation of cAMP-dependent protein kinase by cGMP. Am J Physiol. 1994 Nov;267(5 Pt 1):C1405–C1413. doi: 10.1152/ajpcell.1994.267.5.C1405. [DOI] [PubMed] [Google Scholar]
- Delacrétaz E., Hayoz D., Osterheld M. C., Genton C. Y., Brunner H. R., Waeber B. Long-term nitric oxide synthase inhibition and distensibility of carotid artery in intact rats. Hypertension. 1994 Jun;23(6 Pt 2):967–970. doi: 10.1161/01.hyp.23.6.967. [DOI] [PubMed] [Google Scholar]
- Garg U. C., Hassid A. Nitric oxide-generating vasodilators and 8-bromo-cyclic guanosine monophosphate inhibit mitogenesis and proliferation of cultured rat vascular smooth muscle cells. J Clin Invest. 1989 May;83(5):1774–1777. doi: 10.1172/JCI114081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopalakrishna R., Chen Z. H., Gundimeda U. Nitric oxide and nitric oxide-generating agents induce a reversible inactivation of protein kinase C activity and phorbol ester binding. J Biol Chem. 1993 Dec 25;268(36):27180–27185. [PubMed] [Google Scholar]
- Griffin S. A., Brown W. C., MacPherson F., McGrath J. C., Wilson V. G., Korsgaard N., Mulvany M. J., Lever A. F. Angiotensin II causes vascular hypertrophy in part by a non-pressor mechanism. Hypertension. 1991 May;17(5):626–635. doi: 10.1161/01.hyp.17.5.626. [DOI] [PubMed] [Google Scholar]
- Hwa J. J., Ghibaudi L., Williams P., Chatterjee M. Comparison of acetylcholine-dependent relaxation in large and small arteries of rat mesenteric vascular bed. Am J Physiol. 1994 Mar;266(3 Pt 2):H952–H958. doi: 10.1152/ajpheart.1994.266.3.H952. [DOI] [PubMed] [Google Scholar]
- Jover B., Herizi A., Ventre F., Dupont M., Mimran A. Sodium and angiotensin in hypertension induced by long-term nitric oxide blockade. Hypertension. 1993 Jun;21(6 Pt 2):944–948. doi: 10.1161/01.hyp.21.6.944. [DOI] [PubMed] [Google Scholar]
- Kolpakov V., Gordon D., Kulik T. J. Nitric oxide-generating compounds inhibit total protein and collagen synthesis in cultured vascular smooth muscle cells. Circ Res. 1995 Feb;76(2):305–309. doi: 10.1161/01.res.76.2.305. [DOI] [PubMed] [Google Scholar]
- Levy B. I., Michel J. B., Salzmann J. L., Azizi M., Poitevin P., Safar M., Camilleri J. P. Effects of chronic inhibition of converting enzyme on mechanical and structural properties of arteries in rat renovascular hypertension. Circ Res. 1988 Jul;63(1):227–239. doi: 10.1161/01.res.63.1.227. [DOI] [PubMed] [Google Scholar]
- Manning R. D., Jr, Hu L., Williamson T. D. Mechanisms involved in the cardiovascular-renal actions of nitric oxide inhibition. Hypertension. 1994 Jun;23(6 Pt 2):951–956. doi: 10.1161/01.hyp.23.6.951. [DOI] [PubMed] [Google Scholar]
- Moncada S., Palmer R. M., Higgs E. A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991 Jun;43(2):109–142. [PubMed] [Google Scholar]
- Moncada S., Rees D. D., Schulz R., Palmer R. M. Development and mechanism of a specific supersensitivity to nitrovasodilators after inhibition of vascular nitric oxide synthesis in vivo. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2166–2170. doi: 10.1073/pnas.88.6.2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulvany M. J., Halpern W. Contractile properties of small arterial resistance vessels in spontaneously hypertensive and normotensive rats. Circ Res. 1977 Jul;41(1):19–26. doi: 10.1161/01.res.41.1.19. [DOI] [PubMed] [Google Scholar]
- Nagao T., Illiano S., Vanhoutte P. M. Heterogeneous distribution of endothelium-dependent relaxations resistant to NG-nitro-L-arginine in rats. Am J Physiol. 1992 Oct;263(4 Pt 2):H1090–H1094. doi: 10.1152/ajpheart.1992.263.4.H1090. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Rees D. D., Ashton D. S., Moncada S. L-arginine is the physiological precursor for the formation of nitric oxide in endothelium-dependent relaxation. Biochem Biophys Res Commun. 1988 Jun 30;153(3):1251–1256. doi: 10.1016/s0006-291x(88)81362-7. [DOI] [PubMed] [Google Scholar]
- Pipili-Synetos E., Sakkoula E., Haralabopoulos G., Andriopoulou P., Peristeris P., Maragoudakis M. E. Evidence that nitric oxide is an endogenous antiangiogenic mediator. Br J Pharmacol. 1994 Mar;111(3):894–902. doi: 10.1111/j.1476-5381.1994.tb14822.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollock D. M., Polakowski J. S., Divish B. J., Opgenorth T. J. Angiotensin blockade reverses hypertension during long-term nitric oxide synthase inhibition. Hypertension. 1993 May;21(5):660–666. doi: 10.1161/01.hyp.21.5.660. [DOI] [PubMed] [Google Scholar]
- Pucci M. L., Miller K. B., Dick L. B., Guan H., Lin L., Nasjletti A. Vascular responsiveness to nitric oxide synthesis inhibition in hypertensive rats. Hypertension. 1994 Jun;23(6 Pt 1):744–751. doi: 10.1161/01.hyp.23.6.744. [DOI] [PubMed] [Google Scholar]
- Ribeiro M. O., Antunes E., de Nucci G., Lovisolo S. M., Zatz R. Chronic inhibition of nitric oxide synthesis. A new model of arterial hypertension. Hypertension. 1992 Sep;20(3):298–303. doi: 10.1161/01.hyp.20.3.298. [DOI] [PubMed] [Google Scholar]
- Richard V., Hogie M., Clozel M., Löffler B. M., Thuillez C. In vivo evidence of an endothelin-induced vasopressor tone after inhibition of nitric oxide synthesis in rats. Circulation. 1995 Feb 1;91(3):771–775. doi: 10.1161/01.cir.91.3.771. [DOI] [PubMed] [Google Scholar]
- Vicaut E., Baudry N., Hou X. Nitric oxide-independent response to acetylcholine by terminal arterioles in rat cremaster muscle. J Appl Physiol (1985) 1994 Aug;77(2):526–533. doi: 10.1152/jappl.1994.77.2.526. [DOI] [PubMed] [Google Scholar]
- Waldron G. J., Garland C. J. Contribution of both nitric oxide and a change in membrane potential to acetylcholine-induced relaxation in the rat small mesenteric artery. Br J Pharmacol. 1994 Jul;112(3):831–836. doi: 10.1111/j.1476-5381.1994.tb13154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D. H., Prewitt R. L. Captopril reduces aortic and microvascular growth in hypertensive and normotensive rats. Hypertension. 1990 Jan;15(1):68–77. doi: 10.1161/01.hyp.15.1.68. [DOI] [PubMed] [Google Scholar]
- Wang Y. X., Poon C. I., Pang C. C. In vitro and ex vivo inhibitory effects of L- and D-enantiomers of NG-nitro-arginine on endothelium-dependent relaxation of rat aorta. J Pharmacol Exp Ther. 1993 Apr;265(1):112–119. [PubMed] [Google Scholar]
- Wang Y. X., Poon C. I., Pang C. C. Vascular pharmacodynamics of NG-nitro-L-arginine methyl ester in vitro and in vivo. J Pharmacol Exp Ther. 1993 Dec;267(3):1091–1099. [PubMed] [Google Scholar]
- Wu C. C., Chen S. J., Yen M. H. Different responses to acetylcholine in the presence of nitric oxide inhibitor in rat aortae and mesenteric arteries. Clin Exp Pharmacol Physiol. 1993 Jun;20(6):405–412. doi: 10.1111/j.1440-1681.1993.tb01717.x. [DOI] [PubMed] [Google Scholar]


