Abstract
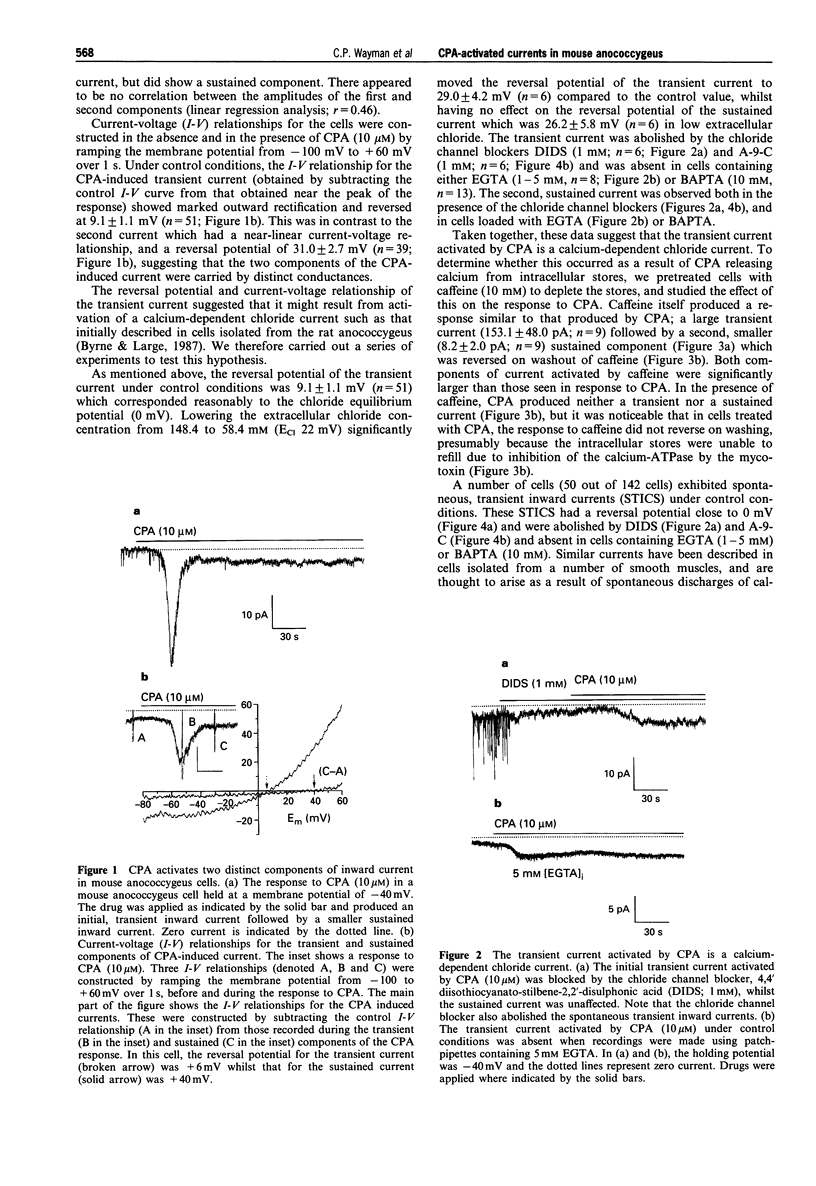
1. By use of the whole-cell configuration of the patch-clamp technique, membrane currents induced by cyclopiazonic acid (CPA; an inhibitor of the sarcoplasmic reticulum (SR) calcium-ATPase) were investigated in single smooth muscle cells freshly dispersed from the mouse anococcygeus. Voltage-dependent calcium currents were blocked with extracellular nifedipine and caesium and tetraethylammonium chloride were used to block voltage-dependent potassium currents. 2. At a holding potential of -40 mV, CPA (10 microM) activated an inward current that consisted of two distinct components. The first was an initial transient current with an amplitude of 19.6 +/- 1.9 pA while the second was sustained and had an amplitude of 3.5 +/- 0.3 pA. 3. The current-voltage (I-V) relationship for the transient current showed marked outward rectification. The current had a reversal potential of 9.1 +/- 1.1 mV which was shifted to 29.0 +/- 4.2 mV when the extracellular chloride concentration was lowered from 148.4 to 58.4 mM. The sustained current had a near-linear I-V relationship and a reversal potential of 31.0 +/- 2.7 mV. Removal of extracellular calcium had no effect on the transient current, but shifted the reversal potential of the sustained current to 18.2 +/- 5.7 mV. 3. The initial transient current was abolished in cells bathed in extracellular solutions containing the chloride channel blockers, 4,4' diisothiocyanato-stilbene-2,2'-disulphonic acid (DIDS; 1 mM) or anthracene-9-carboxylic acid (A-9-C; 1 mM), and was absent in cells containing the calcium buffers EGTA (1 to 5 mM) or BAPTA (10 mM). The second sustained current was unaffected by either the chloride channel blockers or the intracellular calcium buffers. 4. Treatment of the cells with caffeine (10 mM) produced similar inward currents to those produced by CPA. In the presence of caffeine, CPA (10 microM) induced no further inward current. 5. In organ bath studies, CPA (10 microM)-induced contractions of the mouse anococcygeus were inhibited by cadmium and nickel (both 50-400 microM) and the general calcium entry blocker, SKF 96365 (10 microM); lanthanum and gadolinium had no effect at concentrations up to 400 microM. The pharmacology of the CPA-induced non-selective cation current mirrored that of the CPA-induced whole muscle contraction being reversed by cadmium (100 microM) and SKF 96365 (10 microM), but unaffected by lanthanum (400 microM). The initial chloride conductance was unaffected by cadmium, SKF 96365 or lanthanum. 6. It is concluded that CPA activates a transient calcium-dependent chloride current as a consequence of calcium release from intracellular stores; this current would result in depolarization and opening of voltage-operated calcium channels, which mediate the nifedipine-sensitive component of muscle contraction. In addition, as a result of emptying the SR, CPA activates a non-selective cation conductance which may underlie the nifedipine-insensitive calcium entry process utilised during sustained contraction.
Full text
PDF

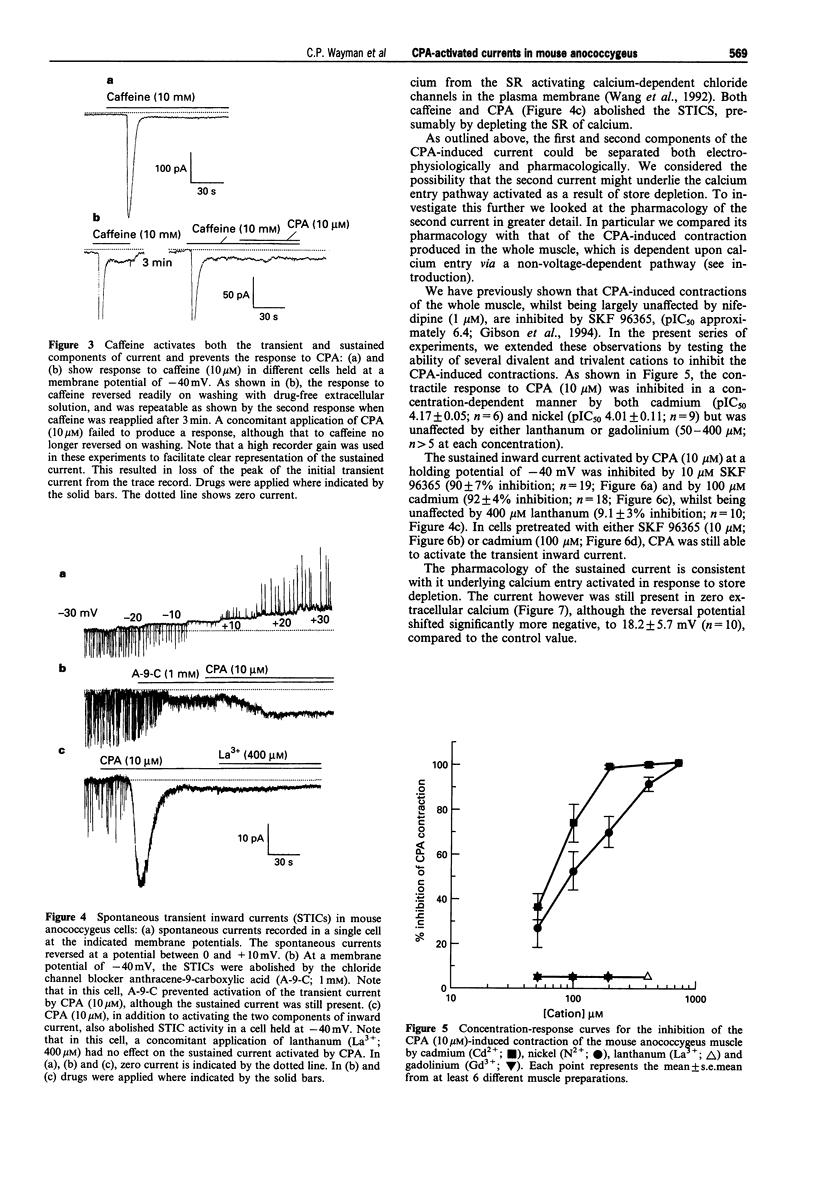


Selected References
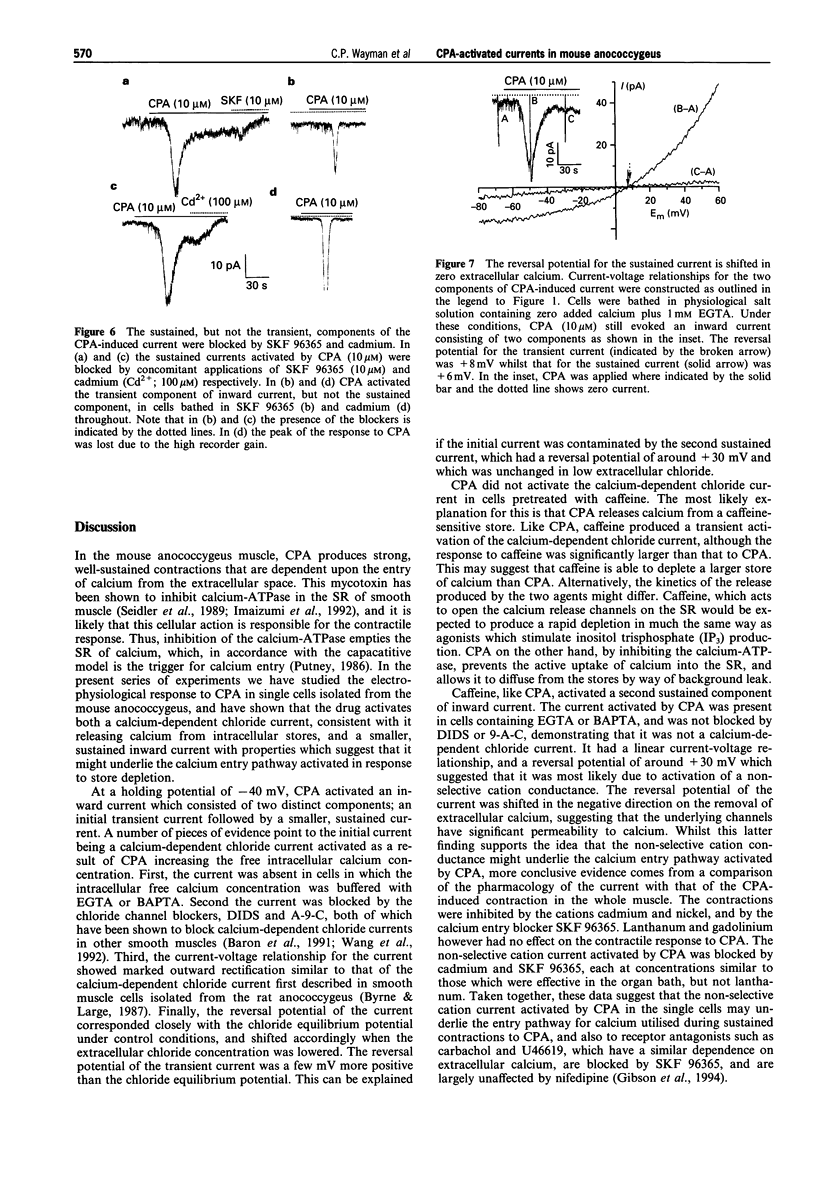
These references are in PubMed. This may not be the complete list of references from this article.
- Amrani Y., Magnier C., Enouf J., Wuytack F., Bronner C. Ca2+ increase and Ca(2+)-influx in human tracheal smooth muscle cells: role of Ca2+ pools controlled by sarco-endoplasmic reticulum Ca(2+)-ATPase 2 isoform. Br J Pharmacol. 1995 Aug;115(7):1204–1210. doi: 10.1111/j.1476-5381.1995.tb15026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron A., Pacaud P., Loirand G., Mironneau C., Mironneau J. Pharmacological block of Ca(2+)-activated Cl- current in rat vascular smooth muscle cells in short-term primary culture. Pflugers Arch. 1991 Dec;419(6):553–558. doi: 10.1007/BF00370294. [DOI] [PubMed] [Google Scholar]
- Byrne N. G., Large W. A. Action of noradrenaline on single smooth muscle cells freshly dispersed from the rat anococcygeus muscle. J Physiol. 1987 Aug;389:513–525. doi: 10.1113/jphysiol.1987.sp016669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demaurex N., Lew D. P., Krause K. H. Cyclopiazonic acid depletes intracellular Ca2+ stores and activates an influx pathway for divalent cations in HL-60 cells. J Biol Chem. 1992 Feb 5;267(4):2318–2324. [PubMed] [Google Scholar]
- Fasolato C., Innocenti B., Pozzan T. Receptor-activated Ca2+ influx: how many mechanisms for how many channels? Trends Pharmacol Sci. 1994 Mar;15(3):77–83. doi: 10.1016/0165-6147(94)90282-8. [DOI] [PubMed] [Google Scholar]
- Felder C. C., Singer-Lahat D., Mathes C. Voltage-independent calcium channels. Regulation by receptors and intracellular calcium stores. Biochem Pharmacol. 1994 Nov 29;48(11):1997–2004. doi: 10.1016/0006-2952(94)90498-7. [DOI] [PubMed] [Google Scholar]
- Gericke M., Droogmans G., Nilius B. Thapsigargin discharges intracellular calcium stores and induces transmembrane currents in human endothelial cells. Pflugers Arch. 1993 Mar;422(6):552–557. doi: 10.1007/BF00374001. [DOI] [PubMed] [Google Scholar]
- Gibson A., McFadzean I., Tucker J. F., Wayman C. Variable potency of nitrergic-nitrovasodilator relaxations of the mouse anococcygeus against different forms of induced tone. Br J Pharmacol. 1994 Dec;113(4):1494–1500. doi: 10.1111/j.1476-5381.1994.tb17165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoth M., Penner R. Calcium release-activated calcium current in rat mast cells. J Physiol. 1993 Jun;465:359–386. doi: 10.1113/jphysiol.1993.sp019681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoth M., Penner R. Depletion of intracellular calcium stores activates a calcium current in mast cells. Nature. 1992 Jan 23;355(6358):353–356. doi: 10.1038/355353a0. [DOI] [PubMed] [Google Scholar]
- Imaizumi Y., Suzuki M., Uyama Y., Muraki K., Watanabe M. Effects of cyclopiazonic acid, a novel Ca(2+)-ATPase inhibitor, on contractile responses and an outward current in smooth muscle. Jpn J Pharmacol. 1992;58 (Suppl 2):401P–401P. [PubMed] [Google Scholar]
- Inoue R. Effect of external Cd2+ and other divalent cations on carbachol-activated non-selective cation channels in guinea-pig ileum. J Physiol. 1991 Oct;442:447–463. doi: 10.1113/jphysiol.1991.sp018802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue R., Isenberg G. Effect of membrane potential on acetylcholine-induced inward current in guinea-pig ileum. J Physiol. 1990 May;424:57–71. doi: 10.1113/jphysiol.1990.sp018055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komori S., Kawai M., Takewaki T., Ohashi H. GTP-binding protein involvement in membrane currents evoked by carbachol and histamine in guinea-pig ileal muscle. J Physiol. 1992 May;450:105–126. doi: 10.1113/jphysiol.1992.sp019118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadzean I., England S. Properties of the inactivating outward current in single smooth muscle cells isolated from the rat anococcygeus. Pflugers Arch. 1992 Jun;421(2-3):117–124. doi: 10.1007/BF00374817. [DOI] [PubMed] [Google Scholar]
- Mendelowitz D., Bacal K., Kunze D. L. Bradykinin-activated calcium influx pathway in bovine aortic endothelial cells. Am J Physiol. 1992 Apr;262(4 Pt 2):H942–H948. doi: 10.1152/ajpheart.1992.262.4.H942. [DOI] [PubMed] [Google Scholar]
- Missiaen L., Declerck I., Droogmans G., Plessers L., De Smedt H., Raeymaekers L., Casteels R. Agonist-dependent Ca2+ and Mn2+ entry dependent on state of filling of Ca2+ stores in aortic smooth muscle cells of the rat. J Physiol. 1990 Aug;427:171–186. doi: 10.1113/jphysiol.1990.sp018166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacaud P., Bolton T. B. Relation between muscarinic receptor cationic current and internal calcium in guinea-pig jejunal smooth muscle cells. J Physiol. 1991 Sep;441:477–499. doi: 10.1113/jphysiol.1991.sp018763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacaud P., Loirand G., Grégoire G., Mironneau C., Mironneau J. Noradrenaline-activated heparin-sensitive Ca2+ entry after depletion of intracellular Ca2+ store in portal vein smooth muscle cells. J Biol Chem. 1993 Feb 25;268(6):3866–3872. [PubMed] [Google Scholar]
- Partridge L. D., Swandulla D. Calcium-activated non-specific cation channels. Trends Neurosci. 1988 Feb;11(2):69–72. doi: 10.1016/0166-2236(88)90167-1. [DOI] [PubMed] [Google Scholar]
- Putney J. W., Jr A model for receptor-regulated calcium entry. Cell Calcium. 1986 Feb;7(1):1–12. doi: 10.1016/0143-4160(86)90026-6. [DOI] [PubMed] [Google Scholar]
- Randriamampita C., Tsien R. Y. Emptying of intracellular Ca2+ stores releases a novel small messenger that stimulates Ca2+ influx. Nature. 1993 Aug 26;364(6440):809–814. doi: 10.1038/364809a0. [DOI] [PubMed] [Google Scholar]
- Seidler N. W., Jona I., Vegh M., Martonosi A. Cyclopiazonic acid is a specific inhibitor of the Ca2+-ATPase of sarcoplasmic reticulum. J Biol Chem. 1989 Oct 25;264(30):17816–17823. [PubMed] [Google Scholar]
- Somasundaram B., Mahaut-Smith M. P. Three cation influx currents activated by purinergic receptor stimulation in rat megakaryocytes. J Physiol. 1994 Oct 15;480(Pt 2):225–231. doi: 10.1113/jphysiol.1994.sp020355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somlyo A. P., Somlyo A. V. Signal transduction and regulation in smooth muscle. Nature. 1994 Nov 17;372(6503):231–236. doi: 10.1038/372231a0. [DOI] [PubMed] [Google Scholar]
- Wang Q., Hogg R. C., Large W. A. Properties of spontaneous inward currents recorded in smooth muscle cells isolated from the rabbit portal vein. J Physiol. 1992;451:525–537. doi: 10.1113/jphysiol.1992.sp019177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q., Large W. A. Noradrenaline-evoked cation conductance recorded with the nystatin whole-cell method in rabbit portal vein cells. J Physiol. 1991 Apr;435:21–39. doi: 10.1113/jphysiol.1991.sp018496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Inazu M., Weir B., Buchanan M., Daniel E. Cyclopiazonic acid stimulates Ca2+ influx through non-specific cation channels in endothelial cells. Eur J Pharmacol. 1994 Jan 14;251(2-3):119–125. doi: 10.1016/0014-2999(94)90391-3. [DOI] [PubMed] [Google Scholar]
- van Breemen C., Saida K. Cellular mechanisms regulating [Ca2+]i smooth muscle. Annu Rev Physiol. 1989;51:315–329. doi: 10.1146/annurev.ph.51.030189.001531. [DOI] [PubMed] [Google Scholar]


