Abstract
1. The purpose of this study was to investigate the topical and systemic anti-hyperalgesic effect of the newly-developed pseudopeptide B2 receptor antagonist, NPC 18688, in different models of nociception in mice. 2. Given systemically 30 min beforehand, NPC 18688 (10-300 nmol kg-1, i.p.) caused no agonist effect, but produced a dose-related and significant inhibition of abdominal constrictions caused by intraperitoneal injection of acetic acid (0.6%), acetylcholine (ACh, 4.5 mg kg-1) or kaolin (50 mg kg-1). The calculated mean ID50s and the percentages of maximal inhibitions (MI) for these effects were: 77, 34 and > 300 nmol kg-1 and 65 +/- 6, 70 +/- 5 and 40 +/- 3%, respectively. The anti-hyperalgesic effect of NPC 18688 (100 nmol kg-1, i.p.) occurred rapidly (30 min) and lasted for at least 150 min. Hoe 140 (3-30 nmol kg-1, i.p.) given 30 min beforehand also inhibited, in a graded manner, acetic acid and ACh-induced writhing, with mean ID50s and MI of 6 and 9 nmol kg-1 and 56 +/- 7 and 62 +/- 6%, respectively. 3. NPC 18688 (10-300 nmol kg-1, i.p.) caused a graded inhibition of both phases of formalin (2.5%)-induced pain, its effects being more potent in relation to the second phase of the formalin test. The calculated mean ID50s and the MI were > 300 and 60 nmol kg-1 and 20 +/- 3 and 60 +/- 5% against the first and second phases of formalin-induced nociception, respectively. NPC 18688 at the same doses also inhibited, in a dose-related manner, formalin-induced paw oedema (MI of 35 +/- 3%). 4. When injected locally in the mouse paw, NPC 18688 (2, 10 and 20 nmol/paw) had no agonist activity. However, when co-injected with formalin NPC 18688 (2-20 nmol/paw), it produced significant inhibition of both phases of formalin response, with MI of 40 +/- 3 and 33 +/- 2%, respectively. NPC 18688 at 10 nmol/paw also significantly inhibited formalin-induced paw oedema (25 +/- 2%). 5. Given intraperitoneally, NPC 18688 (30-300 nmol kg-1) determined a graded inhibition of the nociceptive response caused by intraplantar injection of capsaicin (1.6 micrograms/paw) (40 +/- 2%). However, NPC 18688 (up to 300 nmol kg-1, i.p.), given 30 min beforehand, had no significant analgesic effect when analyzed in the tail flick and in the hot plate pain models, nor did it change the performance of animals in the rota rod test. 6. The action of NPC 18688 was quite selective for the B2 receptor, and like Hoe 140, (1 to 100 nmol kg-1, i.p.) it caused graded inhibition of bradykinin (BK, 3 mol/paw)-induced increase in mouse paw volume, with mean ID50s of 61 and 6 nmol kg-1, respectively. In addition, at 100 nmol kg-1, the dose at which NPC 18688 significantly antagonized BK (3 nmol)-mediated rat paw oedema in naive animals, it had no significant effect on des-Arg9-BK (100 nmol/paw)-induced oedema in paws that had been desensitized to BK. NPC 18688 (210 nmol kg-1), like Hoe 140 (230 nmol kg-1) given s.c. 30 min beforehand, completely abolished BK (28 nmol)-induced hypotension, without affecting the fall of mean arterial blood pressure induced by i.v. injection of ACh (2 nmol kg-1). Finally, NPC 18688 (1 microM) did not affect ACh-mediated contraction in the guinea-pig ileum or toad rectus abdominii in vitro. 7. These results demonstrate that the newly-developed and selective pseudopeptide B2 receptor antagonist, NPC 18688, although less potent than the available second generation of B2 peptide BK receptor antagonists, exhibits topical and long-lasting systemic anti-hyperalgesic properties when analysed in several models of nociception in mice, making it a useful tool for investigating the participation of BK and related kinins in physiological and pathological processes. Finally, this new class of selective pseudopeptide B2 receptor antagonist may constitute a new strategy for developing the third generation of potent and long-lasting orally-active non-peptide BK antagonists, which may be useful for the management of clinical disorders involving BK and relate
Full text
PDF


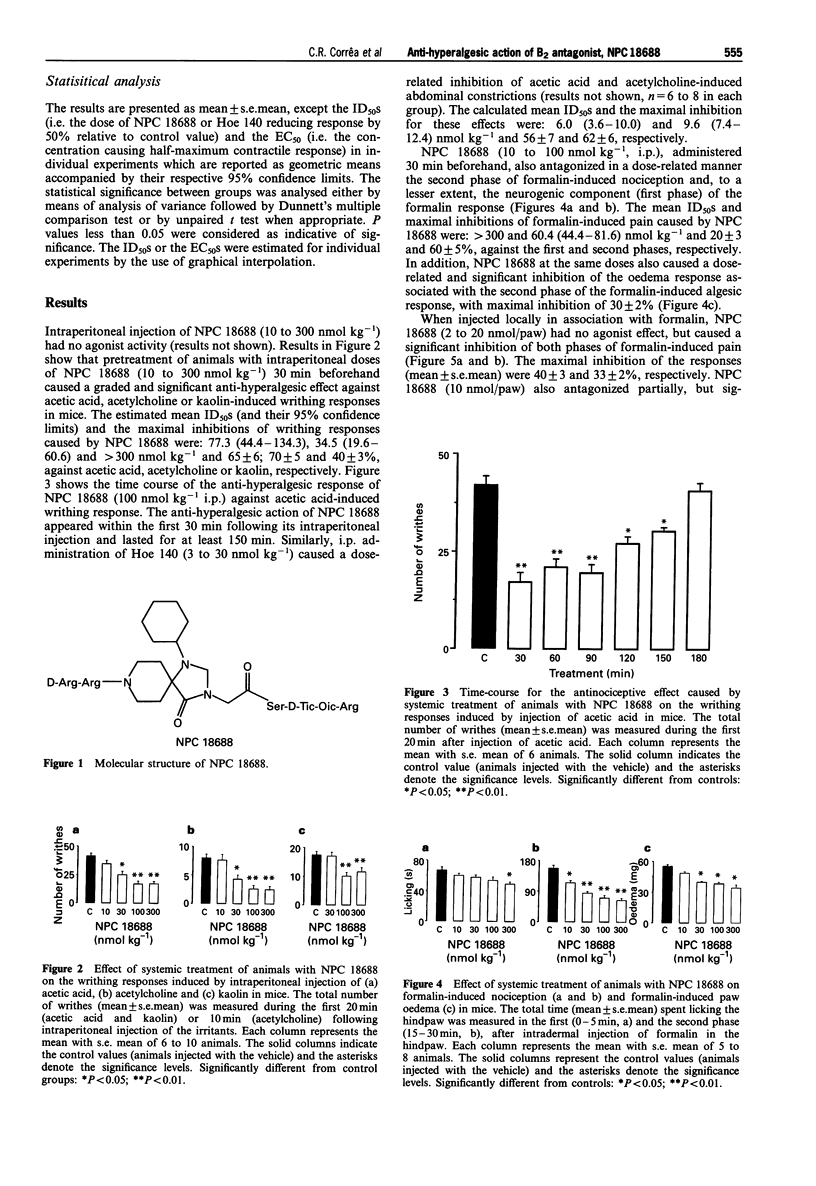


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bentley G. A., Newton S. H., Starr J. Evidence for an action of morphine and the enkephalins on sensory nerve endings in the mouse peritoneum. Br J Pharmacol. 1981 Jun;73(2):325–332. doi: 10.1111/j.1476-5381.1981.tb10425.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhoola K. D., Figueroa C. D., Worthy K. Bioregulation of kinins: kallikreins, kininogens, and kininases. Pharmacol Rev. 1992 Mar;44(1):1–80. [PubMed] [Google Scholar]
- Campos M. M., Calixto J. B. Involvement of B1 and B2 receptors in bradykinin-induced rat paw oedema. Br J Pharmacol. 1995 Mar;114(5):1005–1013. doi: 10.1111/j.1476-5381.1995.tb13305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman V., Dickenson A. H. The spinal and peripheral roles of bradykinin and prostaglandins in nociceptive processing in the rat. Eur J Pharmacol. 1992 Sep 4;219(3):427–433. doi: 10.1016/0014-2999(92)90484-l. [DOI] [PubMed] [Google Scholar]
- Collier H. O., Dinneen L. C., Johnson C. A., Schneider C. The abdominal constriction response and its suppression by analgesic drugs in the mouse. Br J Pharmacol Chemother. 1968 Feb;32(2):295–310. doi: 10.1111/j.1476-5381.1968.tb00973.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrêa C. R., Calixto J. B. Evidence for participation of B1 and B2 kinin receptors in formalin-induced nociceptive response in the mouse. Br J Pharmacol. 1993 Sep;110(1):193–198. doi: 10.1111/j.1476-5381.1993.tb13791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUNHAM N. W., MIYA T. S. A note on a simple apparatus for detecting neurological deficit in rats and mice. J Am Pharm Assoc Am Pharm Assoc. 1957 Mar;46(3):208–209. doi: 10.1002/jps.3030460322. [DOI] [PubMed] [Google Scholar]
- Dray A., Dickenson A. Systemic capsaicin and olvanil reduce the acute algogenic and the late inflammatory phase following formalin injection into rodent paw. Pain. 1991 Oct;47(1):79–83. doi: 10.1016/0304-3959(91)90014-O. [DOI] [PubMed] [Google Scholar]
- Dray A., Perkins M. Bradykinin and inflammatory pain. Trends Neurosci. 1993 Mar;16(3):99–104. doi: 10.1016/0166-2236(93)90133-7. [DOI] [PubMed] [Google Scholar]
- EDDY N. B., LEIMBACH D. Synthetic analgesics. II. Dithienylbutenyl- and dithienylbutylamines. J Pharmacol Exp Ther. 1953 Mar;107(3):385–393. [PubMed] [Google Scholar]
- Eggerickx D., Raspe E., Bertrand D., Vassart G., Parmentier M. Molecular cloning, functional expression and pharmacological characterization of a human bradykinin B2 receptor gene. Biochem Biophys Res Commun. 1992 Sep 30;187(3):1306–1313. doi: 10.1016/0006-291x(92)90445-q. [DOI] [PubMed] [Google Scholar]
- Farmer S. G., Burch R. M. Biochemical and molecular pharmacology of kinin receptors. Annu Rev Pharmacol Toxicol. 1992;32:511–536. doi: 10.1146/annurev.pa.32.040192.002455. [DOI] [PubMed] [Google Scholar]
- Fujiyoshi T., Dozen M., Iida H., Ikeda K., Hayashi I., Oh-ishi S. Demonstration of kinin-release in the peritoneal exudate of kaolin-induced writhing in mice. Jpn J Pharmacol. 1990 Jun;53(2):255–258. doi: 10.1254/jjp.53.255. [DOI] [PubMed] [Google Scholar]
- Haley J. E., Dickenson A. H., Schachter M. Electrophysiological evidence for a role of bradykinin in chemical nociception in the rat. Neurosci Lett. 1989 Feb 13;97(1-2):198–202. doi: 10.1016/0304-3940(89)90163-8. [DOI] [PubMed] [Google Scholar]
- Hall J. M. Bradykinin receptors: pharmacological properties and biological roles. Pharmacol Ther. 1992 Nov;56(2):131–190. doi: 10.1016/0163-7258(92)90016-s. [DOI] [PubMed] [Google Scholar]
- Heapy C. G., Shaw J. S., Farmer S. C. Differential sensitivity of antinociceptive assays to the bradykinin antagonist Hoe 140. Br J Pharmacol. 1993 Jan;108(1):209–213. doi: 10.1111/j.1476-5381.1993.tb13464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess J. F., Borkowski J. A., Macneil T., Stonesifer G. Y., Fraher J., Strader C. D., Ransom R. W. Differential pharmacology of cloned human and mouse B2 bradykinin receptors. Mol Pharmacol. 1994 Jan;45(1):1–8. [PubMed] [Google Scholar]
- Hess J. F., Borkowski J. A., Young G. S., Strader C. D., Ransom R. W. Cloning and pharmacological characterization of a human bradykinin (BK-2) receptor. Biochem Biophys Res Commun. 1992 Apr 15;184(1):260–268. doi: 10.1016/0006-291x(92)91187-u. [DOI] [PubMed] [Google Scholar]
- Hock F. J., Wirth K., Albus U., Linz W., Gerhards H. J., Wiemer G., Henke S., Breipohl G., König W., Knolle J. Hoe 140 a new potent and long acting bradykinin-antagonist: in vitro studies. Br J Pharmacol. 1991 Mar;102(3):769–773. doi: 10.1111/j.1476-5381.1991.tb12248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunskaar S., Hole K. The formalin test in mice: dissociation between inflammatory and non-inflammatory pain. Pain. 1987 Jul;30(1):103–114. doi: 10.1016/0304-3959(87)90088-1. [DOI] [PubMed] [Google Scholar]
- Kindgen-Milles D., Klement W. Pain and inflammation evoked in human skin by bradykinin receptor antagonists. Eur J Pharmacol. 1992 Jul 21;218(1):183–185. doi: 10.1016/0014-2999(92)90164-y. [DOI] [PubMed] [Google Scholar]
- Kyle D. J., Martin J. A., Farmer S. G., Burch R. M. Design and conformational analysis of several highly potent bradykinin receptor antagonists. J Med Chem. 1991 Mar;34(3):1230–1233. doi: 10.1021/jm00107a052. [DOI] [PubMed] [Google Scholar]
- Kyle D. J. Structural features of the bradykinin receptor as determined by computer simulations, mutagenesis experiments, and conformationally constrained ligands: establishing the framework for the design of new antagonists. Braz J Med Biol Res. 1994 Aug;27(8):1757–1779. [PubMed] [Google Scholar]
- Lembeck F., Griesbacher T., Eckhardt M., Henke S., Breipohl G., Knolle J. New, long-acting, potent bradykinin antagonists. Br J Pharmacol. 1991 Feb;102(2):297–304. doi: 10.1111/j.1476-5381.1991.tb12169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lembeck F., Griesbacher T., Eckhardt M., Henke S., Breipohl G., Knolle J. New, long-acting, potent bradykinin antagonists. Br J Pharmacol. 1991 Feb;102(2):297–304. doi: 10.1111/j.1476-5381.1991.tb12169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marceau F., Lussier A., Regoli D., Giroud J. P. Pharmacology of kinins: their relevance to tissue injury and inflammation. Gen Pharmacol. 1983;14(2):209–229. doi: 10.1016/0306-3623(83)90001-0. [DOI] [PubMed] [Google Scholar]
- McEachern A. E., Shelton E. R., Bhakta S., Obernolte R., Bach C., Zuppan P., Fujisaki J., Aldrich R. W., Jarnagin K. Expression cloning of a rat B2 bradykinin receptor. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7724–7728. doi: 10.1073/pnas.88.17.7724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre P., Phillips E., Skidmore E., Brown M., Webb M. Cloned murine bradykinin receptor exhibits a mixed B1 and B2 pharmacological selectivity. Mol Pharmacol. 1993 Aug;44(2):346–355. [PubMed] [Google Scholar]
- Medeiros Y. S., Calixto J. B. Analysis of the mechanisms underlying the biphasic responses to bradykinin in circular muscle from guinea pig ileum. Eur J Pharmacol. 1993 Sep 14;241(2-3):157–163. doi: 10.1016/0014-2999(93)90197-p. [DOI] [PubMed] [Google Scholar]
- Menke J. G., Borkowski J. A., Bierilo K. K., MacNeil T., Derrick A. W., Schneck K. A., Ransom R. W., Strader C. D., Linemeyer D. L., Hess J. F. Expression cloning of a human B1 bradykinin receptor. J Biol Chem. 1994 Aug 26;269(34):21583–21586. [PubMed] [Google Scholar]
- Moore P. K., Oluyomi A. O., Babbedge R. C., Wallace P., Hart S. L. L-NG-nitro arginine methyl ester exhibits antinociceptive activity in the mouse. Br J Pharmacol. 1991 Jan;102(1):198–202. doi: 10.1111/j.1476-5381.1991.tb12153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray C. W., Cowan A., Larson A. A. Neurokinin and NMDA antagonists (but not a kainic acid antagonist) are antinociceptive in the mouse formalin model. Pain. 1991 Feb;44(2):179–185. doi: 10.1016/0304-3959(91)90135-K. [DOI] [PubMed] [Google Scholar]
- Powell S. J., Slynn G., Thomas C., Hopkins B., Briggs I., Graham A. Human bradykinin B2 receptor: nucleotide sequence analysis and assignment to chromosome 14. Genomics. 1993 Feb;15(2):435–438. doi: 10.1006/geno.1993.1084. [DOI] [PubMed] [Google Scholar]
- Proud D., Kaplan A. P. Kinin formation: mechanisms and role in inflammatory disorders. Annu Rev Immunol. 1988;6:49–83. doi: 10.1146/annurev.iy.06.040188.000405. [DOI] [PubMed] [Google Scholar]
- Regoli D., Barabé J. Pharmacology of bradykinin and related kinins. Pharmacol Rev. 1980 Mar;32(1):1–46. [PubMed] [Google Scholar]
- Sakurada T., Katsumata K., Tan-No K., Sakurada S., Kisara K. The capsaicin test in mice for evaluating tachykinin antagonists in the spinal cord. Neuropharmacology. 1992 Dec;31(12):1279–1285. doi: 10.1016/0028-3908(92)90057-v. [DOI] [PubMed] [Google Scholar]
- Sakurada T., Katsumata K., Yogo H., Tan-No K., Sakurada S., Kisara K. Antinociception induced by CP 96,345, a non-peptide NK-1 receptor antagonist, in the mouse formalin and capsaicin tests. Neurosci Lett. 1993 Mar 19;151(2):142–145. doi: 10.1016/0304-3940(93)90006-7. [DOI] [PubMed] [Google Scholar]
- Sawutz D. G., Salvino J. M., Dolle R. E., Casiano F., Ward S. J., Houck W. T., Faunce D. M., Douty B. D., Baizman E., Awad M. M. The nonpeptide WIN 64338 is a bradykinin B2 receptor antagonist. Proc Natl Acad Sci U S A. 1994 May 24;91(11):4693–4697. doi: 10.1073/pnas.91.11.4693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata M., Ohkubo T., Takahashi H., Inoki R. Interaction of bradykinin with substance P on vascular permeability and pain response. Jpn J Pharmacol. 1986 Jul;41(3):427–429. doi: 10.1254/jjp.41.427. [DOI] [PubMed] [Google Scholar]
- Shibata M., Ohkubo T., Takahashi H., Inoki R. Modified formalin test: characteristic biphasic pain response. Pain. 1989 Sep;38(3):347–352. doi: 10.1016/0304-3959(89)90222-4. [DOI] [PubMed] [Google Scholar]
- Steranka L. R., Manning D. C., DeHaas C. J., Ferkany J. W., Borosky S. A., Connor J. R., Vavrek R. J., Stewart J. M., Snyder S. H. Bradykinin as a pain mediator: receptors are localized to sensory neurons, and antagonists have analgesic actions. Proc Natl Acad Sci U S A. 1988 May;85(9):3245–3249. doi: 10.1073/pnas.85.9.3245. [DOI] [PMC free article] [PubMed] [Google Scholar]


