Abstract
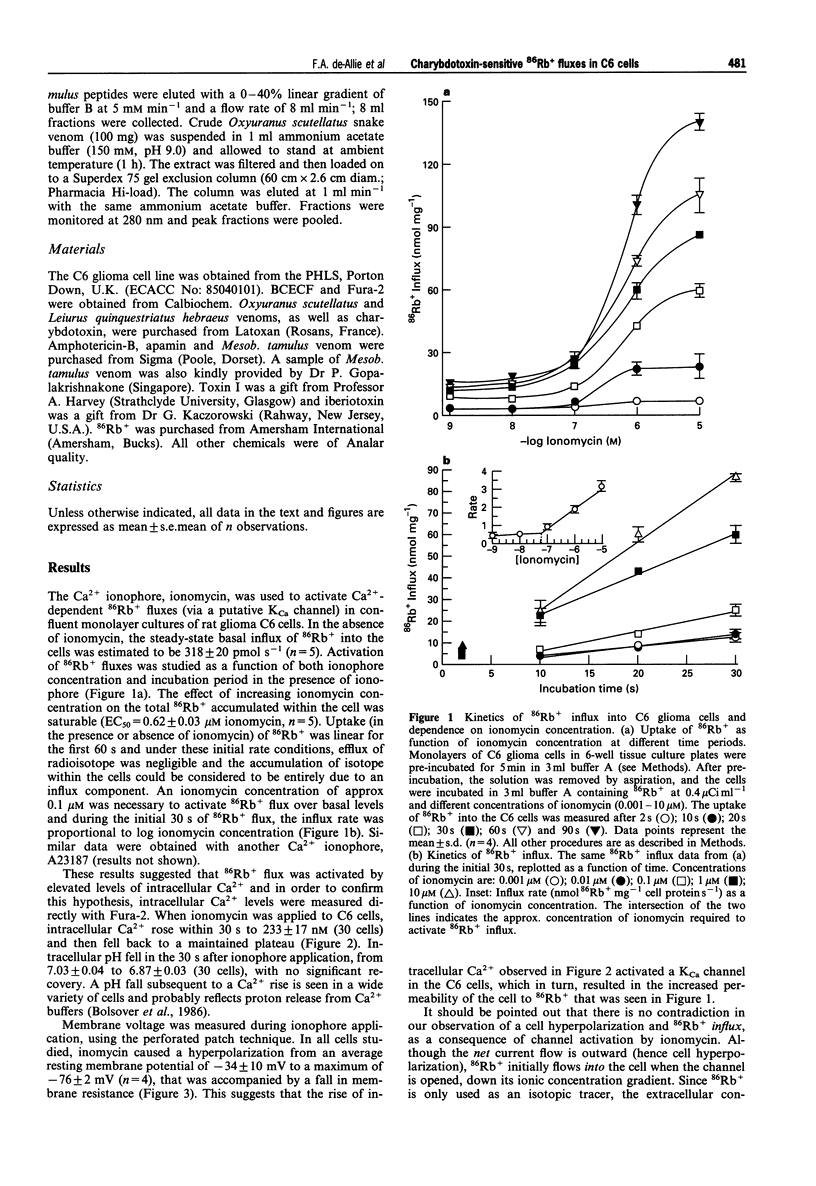
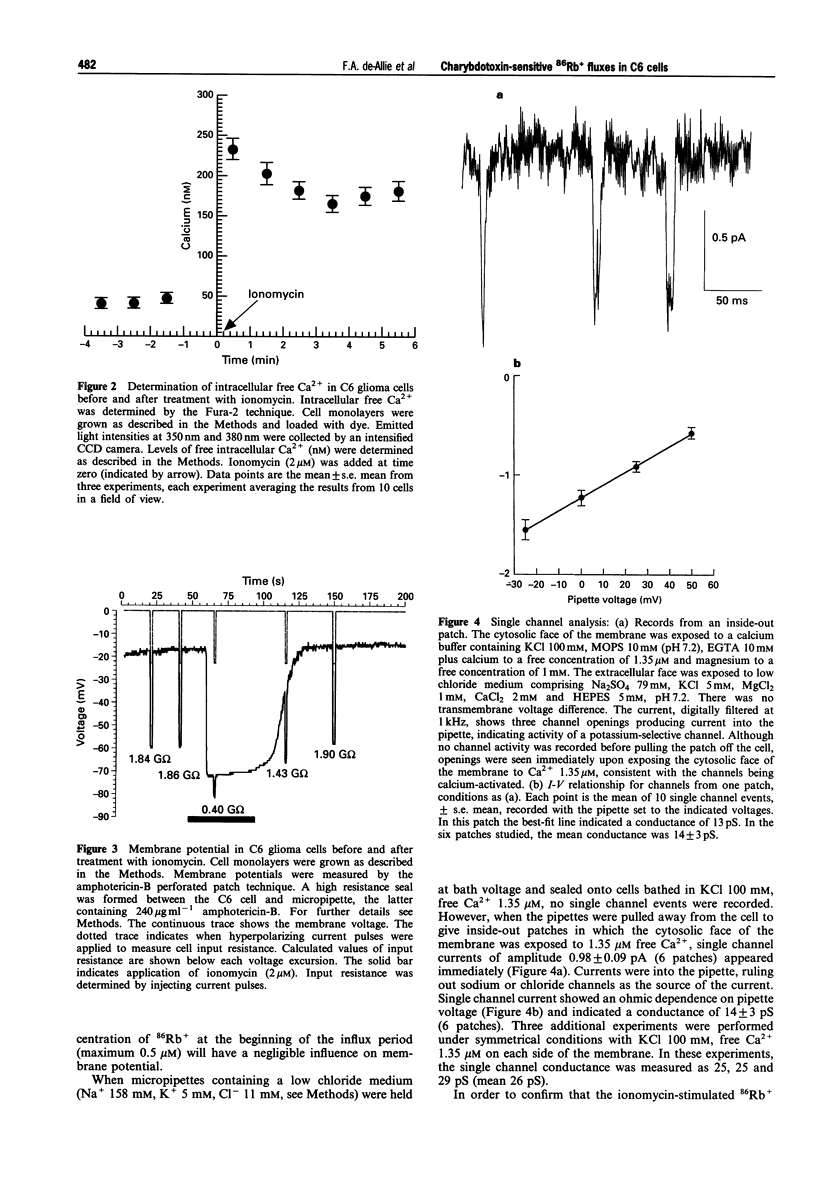
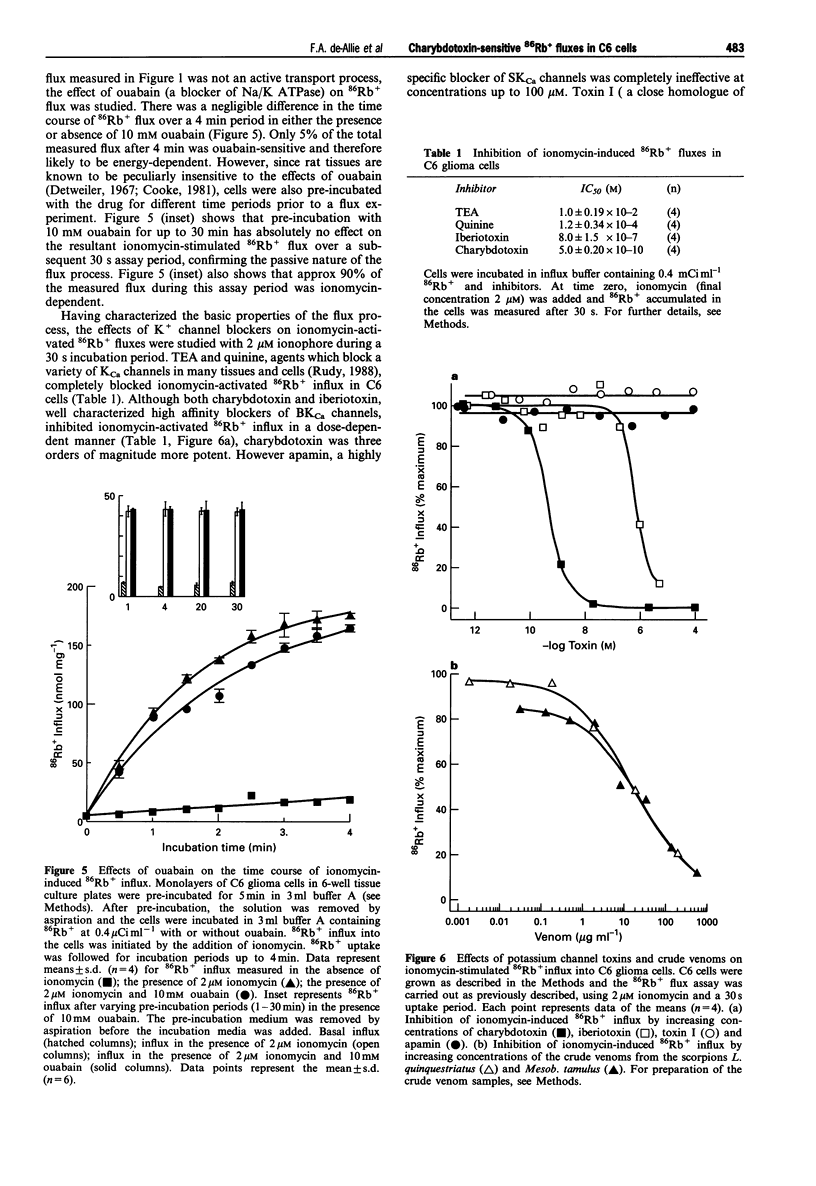
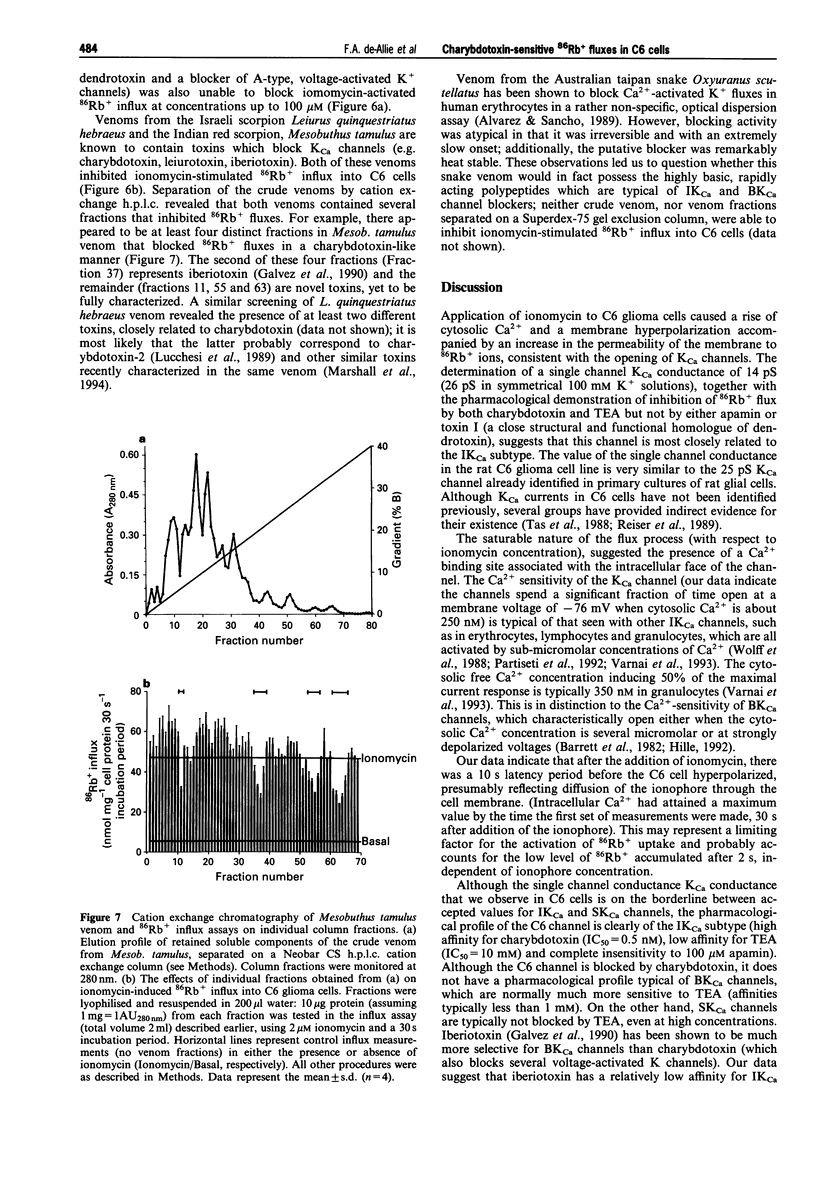
1. The pharmacological characteristics of a putative Ca2+ activated K+ channel (IKCa channel) in rat glioma C6 cells were studied in the presence of the Ca2+ ionophore, ionomycin and various K+ channel blockers, 86Rb+ being used as a radioisotopic tracer for K+. 2. The resting 86Rb+ influx into C6 cells was 318 +/- 20 pmol s-1. The threshold for ionomycin activation of 86Rb+ influx was approx. 100 nM. At ionomycin concentrations above the activation threshold, the initial rate of 86Rb+ influx was proportional to ionophore concentration. Ionomycin-activated 86Rb+ flux was saturable (EC50 = 0.62 +/- 0.03 microM) and was not inhibited by ouabain. 3. Intracellular Ca2+ increased within 30 s from a basal level of 42 +/- 2 nM to 233 +/- 17 nM, after addition of 2 microM ionomycin. During this period, intracellular pH fell from 7.03 +/- 0.04 to 6.87 +/- 0.03 and the cell hyperpolarized from -34 +/- 10 mV to -76 +/- 2 mV. 4. Single channel conductance measurements on inside-out patches in physiological K+ solutions identified a 14 +/- 3 pS CA(2+)-activated K+ current between -25 mV and +50 mV. In symmetrical (100 mM) K+, the single channel conductance was 26 pS. 5. Externally applied quinine (IC50 = 0.12 +/- 0.34 mM) and tetraethylammonium chloride (IC50 = 10 +/- 1.9 mM) inhibited 86Rb+ influx into C6 cells in a concentration-dependent manner. Charybdotoxin (IC50 = 0.5 +/- 0.02 nM) and iberiotoxin (IC50 = 800 +/- 150 nM), as well as the crude venoms from the scorpions Leiurus quinquestriatus and Mesobuthus tamulus, also inhibited 86Rb+ influx. In contrast, apamin and toxin I had no inhibitory effects on 86Rb+ flux. A screen of fractions from cation exchange h.p.l.c. of Mesob. tamulus venom revealed the presence of at least four charybdotoxin-like peptides. One of these was iberiotoxin; the other three are novel toxins. 6. The ionomycin-activated 86Rb+ influx into rat C6 glioma cells has proved to be a valuable pharmacological assay for the screening of toxins and crude venoms which modify intermediate conductance, Ca2+ activated K+ channel activity.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alvarez J., García-Sancho J. Inhibition of red cell Ca2+-dependent K+ channels by snake venoms. Biochim Biophys Acta. 1989 Apr 14;980(2):134–138. doi: 10.1016/0005-2736(89)90391-x. [DOI] [PubMed] [Google Scholar]
- Anderson C. S., MacKinnon R., Smith C., Miller C. Charybdotoxin block of single Ca2+-activated K+ channels. Effects of channel gating, voltage, and ionic strength. J Gen Physiol. 1988 Mar;91(3):317–333. doi: 10.1085/jgp.91.3.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auguste P., Hugues M., Gravé B., Gesquière J. C., Maes P., Tartar A., Romey G., Schweitz H., Lazdunski M. Leiurotoxin I (scyllatoxin), a peptide ligand for Ca2(+)-activated K+ channels. Chemical synthesis, radiolabeling, and receptor characterization. J Biol Chem. 1990 Mar 15;265(8):4753–4759. [PubMed] [Google Scholar]
- Barrett J. N., Magleby K. L., Pallotta B. S. Properties of single calcium-activated potassium channels in cultured rat muscle. J Physiol. 1982 Oct;331:211–230. doi: 10.1113/jphysiol.1982.sp014370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benda P., Lightbody J., Sato G., Levine L., Sweet W. Differentiated rat glial cell strain in tissue culture. Science. 1968 Jul 26;161(3839):370–371. doi: 10.1126/science.161.3839.370. [DOI] [PubMed] [Google Scholar]
- Blatz A. L., Magleby K. L. Single apamin-blocked Ca-activated K+ channels of small conductance in cultured rat skeletal muscle. Nature. 1986 Oct 23;323(6090):718–720. doi: 10.1038/323718a0. [DOI] [PubMed] [Google Scholar]
- Bolsover S. R., Brown J. E., Goldsmith T. H. Intracellular pH of Limulus ventral photoreceptor cells: measurement with phenol red. Soc Gen Physiol Ser. 1986;40:285–310. [PubMed] [Google Scholar]
- Brown C. D., Simmons N. L. K+ transport in 'tight' epithelial monolayers of MDCK cells. Evidence for a calcium-activated K+ channel. Biochim Biophys Acta. 1982 Aug 25;690(1):95–105. doi: 10.1016/0005-2736(82)90243-7. [DOI] [PubMed] [Google Scholar]
- Burgess G. M., Claret M., Jenkinson D. H. Effects of quinine and apamin on the calcium-dependent potassium permeability of mammalian hepatocytes and red cells. J Physiol. 1981 Aug;317:67–90. doi: 10.1113/jphysiol.1981.sp013814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capiod T., Ogden D. C. The properties of calcium-activated potassium ion channels in guinea-pig isolated hepatocytes. J Physiol. 1989 Feb;409:285–295. doi: 10.1113/jphysiol.1989.sp017497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castle N. A., Strong P. N. Identification of two toxins from scorpion (Leiurus quinquestriatus) venom which block distinct classes of calcium-activated potassium channel. FEBS Lett. 1986 Dec 1;209(1):117–121. doi: 10.1016/0014-5793(86)81095-x. [DOI] [PubMed] [Google Scholar]
- Chicchi G. G., Gimenez-Gallego G., Ber E., Garcia M. L., Winquist R., Cascieri M. A. Purification and characterization of a unique, potent inhibitor of apamin binding from Leiurus quinquestriatus hebraeus venom. J Biol Chem. 1988 Jul 25;263(21):10192–10197. [PubMed] [Google Scholar]
- Cooke K. R. Ouabain and regulation of cellular volume in slices of mammalian renal cortex. J Physiol. 1981 Nov;320:319–332. doi: 10.1113/jphysiol.1981.sp013952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crest M., Jacquet G., Gola M., Zerrouk H., Benslimane A., Rochat H., Mansuelle P., Martin-Eauclaire M. F. Kaliotoxin, a novel peptidyl inhibitor of neuronal BK-type Ca(2+)-activated K+ channels characterized from Androctonus mauretanicus mauretanicus venom. J Biol Chem. 1992 Jan 25;267(3):1640–1647. [PubMed] [Google Scholar]
- Detweiler D. K. Comparative pharmacology of cardiac glycosides. Fed Proc. 1967 Jul-Aug;26(4):1119–1124. [PubMed] [Google Scholar]
- Erecińska M., Dagani F., Nelson D., Deas J., Silver I. A. Relations between intracellular ions and energy metabolism: a study with monensin in synaptosomes, neurons, and C6 glioma cells. J Neurosci. 1991 Aug;11(8):2410–2421. doi: 10.1523/JNEUROSCI.11-08-02410.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvez A., Gimenez-Gallego G., Reuben J. P., Roy-Contancin L., Feigenbaum P., Kaczorowski G. J., Garcia M. L. Purification and characterization of a unique, potent, peptidyl probe for the high conductance calcium-activated potassium channel from venom of the scorpion Buthus tamulus. J Biol Chem. 1990 Jul 5;265(19):11083–11090. [PubMed] [Google Scholar]
- Garcia-Calvo M., Leonard R. J., Novick J., Stevens S. P., Schmalhofer W., Kaczorowski G. J., Garcia M. L. Purification, characterization, and biosynthesis of margatoxin, a component of Centruroides margaritatus venom that selectively inhibits voltage-dependent potassium channels. J Biol Chem. 1993 Sep 5;268(25):18866–18874. [PubMed] [Google Scholar]
- Garcia-Calvo M., Vázquez J., Smith M., Kaczorowski G. J., Garcia M. L. Characterization of the solubilized charybdotoxin receptor from bovine aortic smooth muscle. Biochemistry. 1991 Nov 19;30(46):11157–11164. doi: 10.1021/bi00110a020. [DOI] [PubMed] [Google Scholar]
- Garcia M. L., Garcia-Calvo M., Hidalgo P., Lee A., MacKinnon R. Purification and characterization of three inhibitors of voltage-dependent K+ channels from Leiurus quinquestriatus var. hebraeus venom. Biochemistry. 1994 Jun 7;33(22):6834–6839. doi: 10.1021/bi00188a012. [DOI] [PubMed] [Google Scholar]
- Gardner-Medwin A. R. Analysis of potassium dynamics in mammalian brain tissue. J Physiol. 1983 Feb;335:393–426. doi: 10.1113/jphysiol.1983.sp014541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauldie J., Hanson J. M., Rumjanek F. D., Shipolini R. A., Vernon C. A. The peptide components of bee venom. Eur J Biochem. 1976 Jan 15;61(2):369–376. doi: 10.1111/j.1432-1033.1976.tb10030.x. [DOI] [PubMed] [Google Scholar]
- Gimenez-Gallego G., Navia M. A., Reuben J. P., Katz G. M., Kaczorowski G. J., Garcia M. L. Purification, sequence, and model structure of charybdotoxin, a potent selective inhibitor of calcium-activated potassium channels. Proc Natl Acad Sci U S A. 1988 May;85(10):3329–3333. doi: 10.1073/pnas.85.10.3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey A. L., Marshall D. L., De-Allie F. A., Strong P. N. Interactions between dendrotoxin, a blocker of voltage-dependent potassium channels, and charybdotoxin, a blocker of calcium-activated potassium channels, at binding sites on neuronal membranes. Biochem Biophys Res Commun. 1989 Aug 30;163(1):394–397. doi: 10.1016/0006-291x(89)92148-7. [DOI] [PubMed] [Google Scholar]
- Hermann A., Erxleben C. Charybdotoxin selectively blocks small Ca-activated K channels in Aplysia neurons. J Gen Physiol. 1987 Jul;90(1):27–47. doi: 10.1085/jgp.90.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann A., Hartung K. Ca2+ activated K+ conductance in molluscan neurones. Cell Calcium. 1983 Dec;4(5-6):387–405. doi: 10.1016/0143-4160(83)90016-7. [DOI] [PubMed] [Google Scholar]
- Hugues M., Schmid H., Lazdunski M. Identification of a protein component of the Ca2+-dependent K+ channel by affinity labelling with apamin. Biochem Biophys Res Commun. 1982 Aug 31;107(4):1577–1582. doi: 10.1016/s0006-291x(82)80180-0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Latorre R., Oberhauser A., Labarca P., Alvarez O. Varieties of calcium-activated potassium channels. Annu Rev Physiol. 1989;51:385–399. doi: 10.1146/annurev.ph.51.030189.002125. [DOI] [PubMed] [Google Scholar]
- Lucchesi K., Ravindran A., Young H., Moczydlowski E. Analysis of the blocking activity of charybdotoxin homologs and iodinated derivatives against Ca2+-activated K+ channels. J Membr Biol. 1989 Aug;109(3):269–281. doi: 10.1007/BF01870284. [DOI] [PubMed] [Google Scholar]
- MacKinnon R., Miller C. Mutant potassium channels with altered binding of charybdotoxin, a pore-blocking peptide inhibitor. Science. 1989 Sep 22;245(4924):1382–1385. doi: 10.1126/science.2476850. [DOI] [PubMed] [Google Scholar]
- MacVicar B. A. Voltage-dependent calcium channels in glial cells. Science. 1984 Dec 14;226(4680):1345–1347. doi: 10.1126/science.6095454. [DOI] [PubMed] [Google Scholar]
- Marshall D. L., Vatanpour H., Harvey A. L., Boyot P., Pinkasfeld S., Doljansky Y., Bouet F., Ménez A. Neuromuscular effects of some potassium channel blocking toxins from the venom of the scorpion Leiurus quinquestriatus hebreus. Toxicon. 1994 Nov;32(11):1433–1443. doi: 10.1016/0041-0101(94)90415-4. [DOI] [PubMed] [Google Scholar]
- Miller C., Moczydlowski E., Latorre R., Phillips M. Charybdotoxin, a protein inhibitor of single Ca2+-activated K+ channels from mammalian skeletal muscle. Nature. 1985 Jan 24;313(6000):316–318. doi: 10.1038/313316a0. [DOI] [PubMed] [Google Scholar]
- Park Y. B. Ion selectivity and gating of small conductance Ca(2+)-activated K+ channels in cultured rat adrenal chromaffin cells. J Physiol. 1994 Dec 15;481(Pt 3):555–570. doi: 10.1113/jphysiol.1994.sp020463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partiseti M., Choquet D., Diu A., Korn H. Differential regulation of voltage- and calcium-activated potassium channels in human B lymphocytes. J Immunol. 1992 Jun 1;148(11):3361–3368. [PubMed] [Google Scholar]
- Petersen O. H., Maruyama Y. Calcium-activated potassium channels and their role in secretion. Nature. 1984 Feb 23;307(5953):693–696. doi: 10.1038/307693a0. [DOI] [PubMed] [Google Scholar]
- Poenie M. Alteration of intracellular Fura-2 fluorescence by viscosity: a simple correction. Cell Calcium. 1990 Feb-Mar;11(2-3):85–91. doi: 10.1016/0143-4160(90)90062-y. [DOI] [PubMed] [Google Scholar]
- Quandt F. N., MacVicar B. A. Calcium activated potassium channels in cultured astrocytes. Neuroscience. 1986 Sep;19(1):29–41. doi: 10.1016/0306-4522(86)90003-5. [DOI] [PubMed] [Google Scholar]
- Rae J., Cooper K., Gates P., Watsky M. Low access resistance perforated patch recordings using amphotericin B. J Neurosci Methods. 1991 Mar;37(1):15–26. doi: 10.1016/0165-0270(91)90017-t. [DOI] [PubMed] [Google Scholar]
- Rehm H., Lazdunski M. Purification and subunit structure of a putative K+-channel protein identified by its binding properties for dendrotoxin I. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4919–4923. doi: 10.1073/pnas.85.13.4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiser G., Binmöller F. J., Strong P. N., Hamprecht B. Activation of a K+ conductance by bradykinin and by inositol-1,4,5-trisphosphate in rat glioma cells: involvement of intracellular and extracellular Ca2+. Brain Res. 1990 Jan 8;506(2):205–214. doi: 10.1016/0006-8993(90)91252-c. [DOI] [PubMed] [Google Scholar]
- Rudy B. Diversity and ubiquity of K channels. Neuroscience. 1988 Jun;25(3):729–749. doi: 10.1016/0306-4522(88)90033-4. [DOI] [PubMed] [Google Scholar]
- Sabatier J. M., Zerrouk H., Darbon H., Mabrouk K., Benslimane A., Rochat H., Martin-Eauclaire M. F., Van Rietschoten J. P05, a new leiurotoxin I-like scorpion toxin: synthesis and structure-activity relationships of the alpha-amidated analog, a ligand of Ca(2+)-activated K+ channels with increased affinity. Biochemistry. 1993 Mar 23;32(11):2763–2770. doi: 10.1021/bi00062a005. [DOI] [PubMed] [Google Scholar]
- Schweitz H., Bidard J. N., Maes P., Lazdunski M. Charybdotoxin is a new member of the K+ channel toxin family that includes dendrotoxin I and mast cell degranulating peptide. Biochemistry. 1989 Dec 12;28(25):9708–9714. doi: 10.1021/bi00451a025. [DOI] [PubMed] [Google Scholar]
- Schweitz H., Stansfeld C. E., Bidard J. N., Fagni L., Maes P., Lazdunski M. Charybdotoxin blocks dendrotoxin-sensitive voltage-activated K+ channels. FEBS Lett. 1989 Jul 3;250(2):519–522. doi: 10.1016/0014-5793(89)80788-4. [DOI] [PubMed] [Google Scholar]
- Scott V. E., Parcej D. N., Keen J. N., Findlay J. B., Dolly J. O. Alpha-dendrotoxin acceptor from bovine brain is a K+ channel protein. Evidence from the N-terminal sequence of its larger subunit. J Biol Chem. 1990 Nov 25;265(33):20094–20097. [PubMed] [Google Scholar]
- Strong P. N. Potassium channel toxins. Pharmacol Ther. 1990;46(1):137–162. doi: 10.1016/0163-7258(90)90040-9. [DOI] [PubMed] [Google Scholar]
- Strong P. N., Weir S. W., Beech D. J., Hiestand P., Kocher H. P. Effects of potassium channel toxins from Leiurus quinquestriatus hebraeus venom on responses to cromakalim in rabbit blood vessels. Br J Pharmacol. 1989 Nov;98(3):817–826. doi: 10.1111/j.1476-5381.1989.tb14610.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tas P. W., Kress H. G., Koschel K. Presence of a charybdotoxin sensitive Ca2+-activated K+ channel in rat glioma C6 cells. Neurosci Lett. 1988 Dec 5;94(3):279–284. doi: 10.1016/0304-3940(88)90031-6. [DOI] [PubMed] [Google Scholar]
- Tas P. W., Kress H. G., Koschel K. Volatile anesthetics inhibit the ion flux through Ca2+-activated K+ channels of rat glioma C6 cells. Biochim Biophys Acta. 1989 Aug 7;983(2):264–268. doi: 10.1016/0005-2736(89)90243-5. [DOI] [PubMed] [Google Scholar]
- Trachtenberg M. C., Pollen D. A. Neuroglia: biophysical properties and physiologic function. Science. 1970 Feb 27;167(3922):1248–1252. doi: 10.1126/science.167.3922.1248. [DOI] [PubMed] [Google Scholar]
- Varnai P., Demaurex N., Jaconi M., Schlegel W., Lew D. P., Krause K. H. Highly co-operative Ca2+ activation of intermediate-conductance K+ channels in granulocytes from a human cell line. J Physiol. 1993 Dec;472:373–390. doi: 10.1113/jphysiol.1993.sp019952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vázquez J., Feigenbaum P., Katz G., King V. F., Reuben J. P., Roy-Contancin L., Slaughter R. S., Kaczorowski G. J., Garcia M. L. Characterization of high affinity binding sites for charybdotoxin in sarcolemmal membranes from bovine aortic smooth muscle. Evidence for a direct association with the high conductance calcium-activated potassium channel. J Biol Chem. 1989 Dec 15;264(35):20902–20909. [PubMed] [Google Scholar]
- Wang S. Y., Castle N. A., Wang G. K. Identification of RBK1 potassium channels in C6 astrocytoma cells. Glia. 1992;5(2):146–153. doi: 10.1002/glia.440050209. [DOI] [PubMed] [Google Scholar]
- Wolff D., Cecchi X., Spalvins A., Canessa M. Charybdotoxin blocks with high affinity the Ca-activated K+ channel of Hb A and Hb S red cells: individual differences in the number of channels. J Membr Biol. 1988 Dec;106(3):243–252. doi: 10.1007/BF01872162. [DOI] [PubMed] [Google Scholar]


