Abstract
1. Human GABAA receptors containing different alpha and beta subunits with a gamma 2s subunit were expressed in Xenopus oocytes and the effects of pentobarbitone on these subunit combinations were examined by electrophysiological recording of GABA currents with the two-electrode voltage-clamp method. 2. Pentobarbitone has previously been shown to have three actions on GABAA receptors: a potentiation of GABA responses, a direct activation of GABAA receptors and, at high concentrations, a block of the GABA chloride channel. In this study pentobarbitone activity consisted of the above mentioned three components on all the subunit combinations tested. However, the affinities and efficacies varied with receptor subtype. 3. Potentiation of GABA by pentobarbitone occurred over the same concentration-range for all the subunits with affinities in the range of 20-35 microM. The degree of potentiation obtained, however, varied from 236% of GABA EC20 on alpha 1 beta 2 gamma 2s to 536% on alpha 6 beta 2 gamma 2s. 4. Examination of the direct effect of pentobarbitone revealed that the type of alpha subunit present determines both the degree of affinity and efficacy obtained. Receptors containing an alpha 6 subunit produced maximum direct responses to pentobarbitone larger than that obtainable with maximum GABA (150% to 170% of maximum GABA). The maximum direct pentobarbitone response obtainable with other alpha subunits ranged between 45% of maximum GABA for alpha 5 beta 2 gamma 2s to 82% for alpha 2 beta 2 gamma 2s. The affinity of the direct action of pentobarbitone on alpha 6 beta 2 gamma 2s was 58 microM compared to affinities for the other alpha subunits ranging from 139 microM on alpha 2 beta 2 gamma 2s to 528 microM on alpha 5 beta 2 gamma 2s. 5. The type of beta subunit present did not influence the direct action of pentobarbitone to the same extent as the alpha subunit. There were no significant differences between affinity or efficacy on oocytes expressing alpha 6 and gamma 2s with beta 1, beta 2 or beta 3. Affinities and efficacies on oocytes expressing alpha 1 and gamma 2s with beta 1, beta 2 or beta 3 were significantly different with pentobarbitone having a higher affinity and efficacy on alpha 1 beta 3 gamma 2s followed by alpha 1 beta 2 gamma 2s and then alpha 1 beta 1 gamma 2s. 6. The direct effect of pentobarbitone was blocked by picrotoxin but not by competitive antagonists, such as bicuculline or SR95531, indicating that the direct agonist activity of pentobarbitone was not mediated via the GABA binding site. 7. For the first time the influence of the various alpha and beta subunits on the effects of pentobarbitone were demonstrated. The results indicate that GABAA receptors containing alpha 6 subunits have both a higher affinity and efficacy for direct activation by pentobarbitone, and reveal that pentobarbitone binds to more than one site on the GABAA receptor, and these are dependent on receptor subunit composition.
Full text
PDF
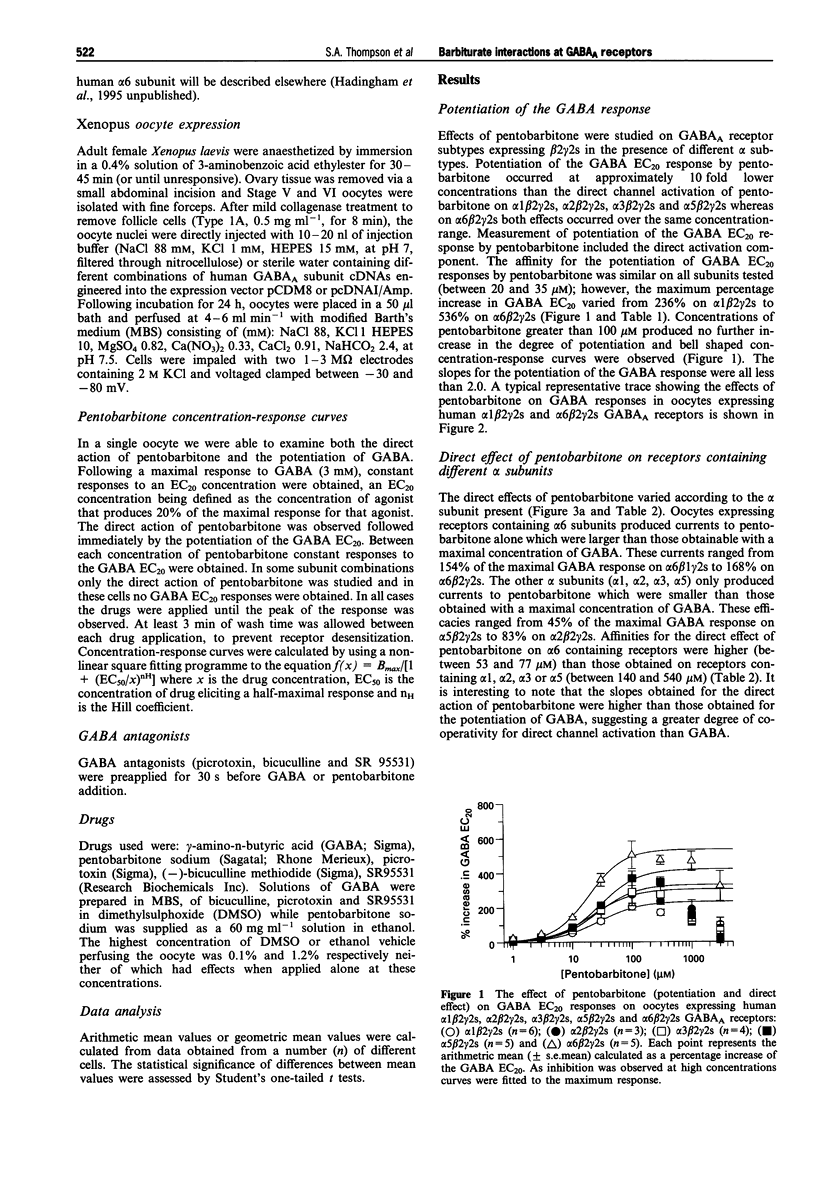
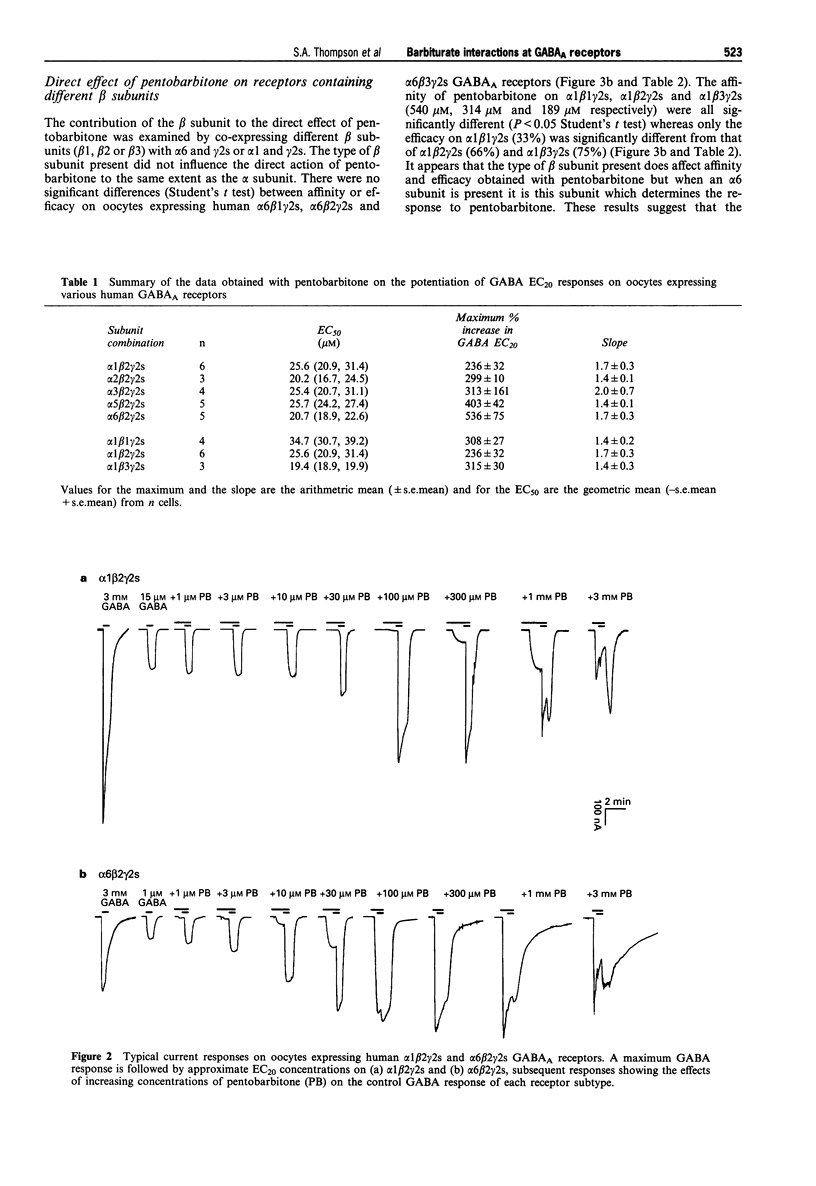
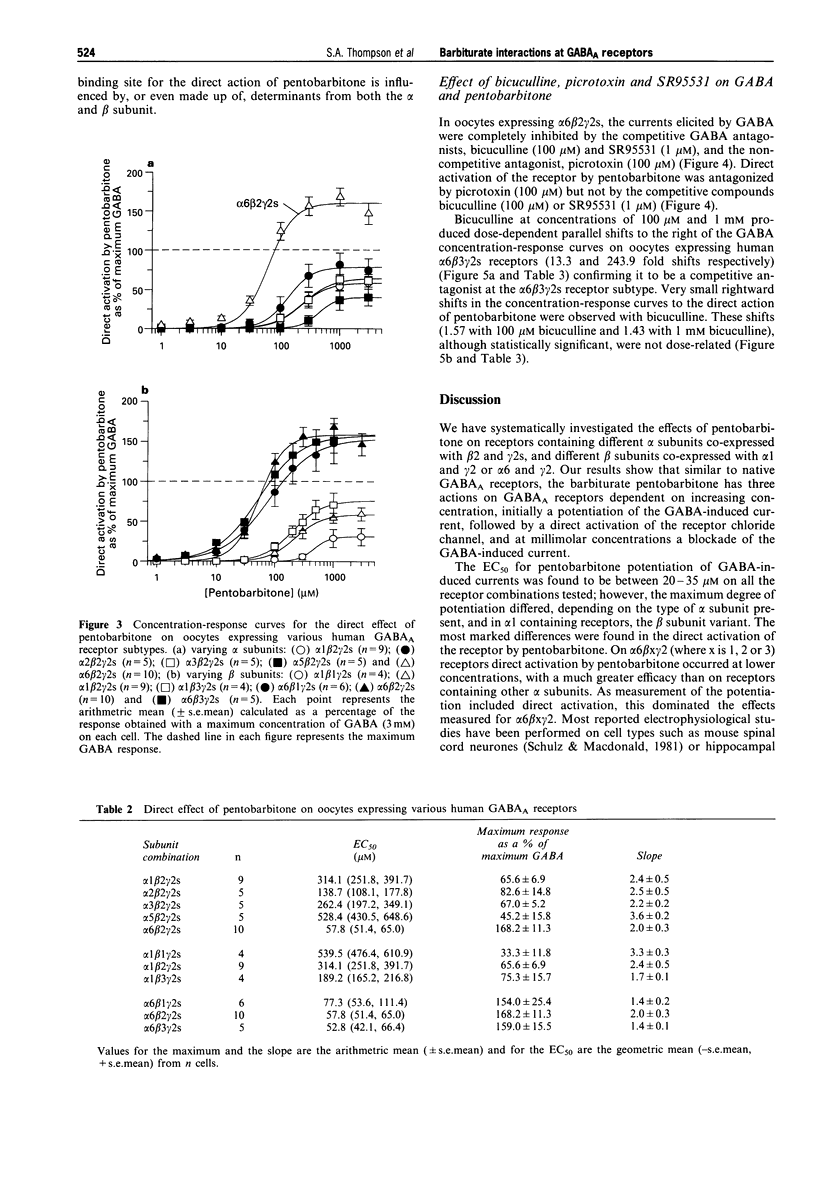


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
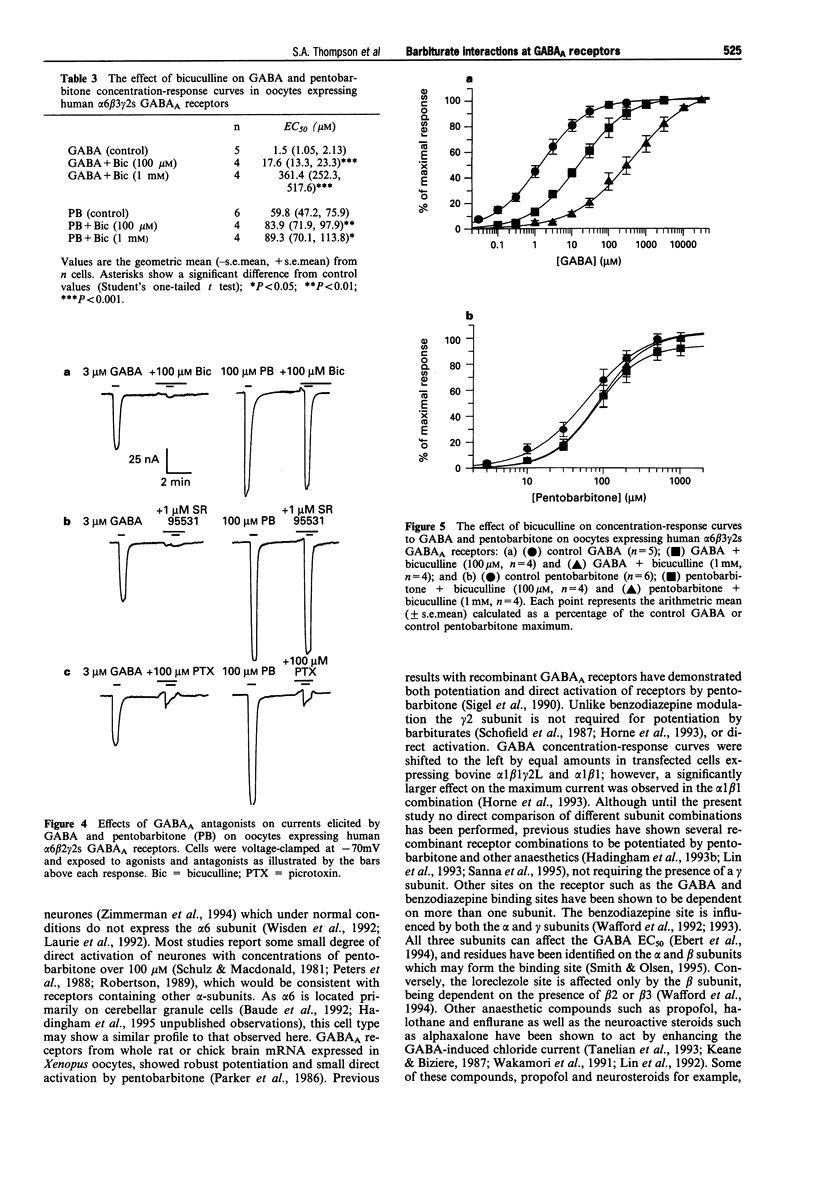
- Akaike N., Maruyama T., Tokutomi N. Kinetic properties of the pentobarbitone-gated chloride current in frog sensory neurones. J Physiol. 1987 Dec;394:85–98. doi: 10.1113/jphysiol.1987.sp016861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amin J., Weiss D. S. GABAA receptor needs two homologous domains of the beta-subunit for activation by GABA but not by pentobarbital. Nature. 1993 Dec 9;366(6455):565–569. doi: 10.1038/366565a0. [DOI] [PubMed] [Google Scholar]
- Baude A., Sequier J. M., McKernan R. M., Olivier K. R., Somogyi P. Differential subcellular distribution of the alpha 6 subunit versus the alpha 1 and beta 2/3 subunits of the GABAA/benzodiazepine receptor complex in granule cells of the cerebellar cortex. Neuroscience. 1992 Dec;51(4):739–748. doi: 10.1016/0306-4522(92)90513-2. [DOI] [PubMed] [Google Scholar]
- Ebert B., Wafford K. A., Whiting P. J., Krogsgaard-Larsen P., Kemp J. A. Molecular pharmacology of gamma-aminobutyric acid type A receptor agonists and partial agonists in oocytes injected with different alpha, beta, and gamma receptor subunit combinations. Mol Pharmacol. 1994 Nov;46(5):957–963. [PubMed] [Google Scholar]
- Evans R. H. Potentiation of the effects of GABA by pentobarbitone. Brain Res. 1979 Jul 27;171(1):113–120. doi: 10.1016/0006-8993(79)90736-4. [DOI] [PubMed] [Google Scholar]
- Franks N. P., Lieb W. R. Molecular and cellular mechanisms of general anaesthesia. Nature. 1994 Feb 17;367(6464):607–614. doi: 10.1038/367607a0. [DOI] [PubMed] [Google Scholar]
- Hadingham K. L., Wingrove P. B., Wafford K. A., Bain C., Kemp J. A., Palmer K. J., Wilson A. W., Wilcox A. S., Sikela J. M., Ragan C. I. Role of the beta subunit in determining the pharmacology of human gamma-aminobutyric acid type A receptors. Mol Pharmacol. 1993 Dec;44(6):1211–1218. [PubMed] [Google Scholar]
- Hadingham K. L., Wingrove P., Le Bourdelles B., Palmer K. J., Ragan C. I., Whiting P. J. Cloning of cDNA sequences encoding human alpha 2 and alpha 3 gamma-aminobutyric acidA receptor subunits and characterization of the benzodiazepine pharmacology of recombinant alpha 1-, alpha 2-, alpha 3-, and alpha 5-containing human gamma-aminobutyric acidA receptors. Mol Pharmacol. 1993 Jun;43(6):970–975. [PubMed] [Google Scholar]
- Hara M., Kai Y., Ikemoto Y. Propofol activates GABAA receptor-chloride ionophore complex in dissociated hippocampal pyramidal neurons of the rat. Anesthesiology. 1993 Oct;79(4):781–788. doi: 10.1097/00000542-199310000-00021. [DOI] [PubMed] [Google Scholar]
- Horne A. L., Harkness P. C., Hadingham K. L., Whiting P., Kemp J. A. The influence of the gamma 2L subunit on the modulation of responses to GABAA receptor activation. Br J Pharmacol. 1993 Mar;108(3):711–716. doi: 10.1111/j.1476-5381.1993.tb12866.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson M. B., Lecar H., Mathers D. A., Barker J. L. Single channel currents activated by gamma-aminobutyric acid, muscimol, and (-)-pentobarbital in cultured mouse spinal neurons. J Neurosci. 1982 Jul;2(7):889–894. doi: 10.1523/JNEUROSCI.02-07-00889.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keane P. E., Biziere K. The effects of general anaesthetics on GABAergic synaptic transmission. Life Sci. 1987 Sep 21;41(12):1437–1448. doi: 10.1016/0024-3205(87)90708-9. [DOI] [PubMed] [Google Scholar]
- Laurie D. J., Seeburg P. H., Wisden W. The distribution of 13 GABAA receptor subunit mRNAs in the rat brain. II. Olfactory bulb and cerebellum. J Neurosci. 1992 Mar;12(3):1063–1076. doi: 10.1523/JNEUROSCI.12-03-01063.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L. H., Chen L. L., Zirrolli J. A., Harris R. A. General anesthetics potentiate gamma-aminobutyric acid actions on gamma-aminobutyric acidA receptors expressed by Xenopus oocytes: lack of involvement of intracellular calcium. J Pharmacol Exp Ther. 1992 Nov;263(2):569–578. [PubMed] [Google Scholar]
- Lin L. H., Whiting P., Harris R. A. Molecular determinants of general anesthetic action: role of GABAA receptor structure. J Neurochem. 1993 Apr;60(4):1548–1553. doi: 10.1111/j.1471-4159.1993.tb03320.x. [DOI] [PubMed] [Google Scholar]
- Léna C., Changeux J. P. Allosteric modulations of the nicotinic acetylcholine receptor. Trends Neurosci. 1993 May;16(5):181–186. doi: 10.1016/0166-2236(93)90150-k. [DOI] [PubMed] [Google Scholar]
- MacDonald R. L., Rogers C. J., Twyman R. E. Barbiturate regulation of kinetic properties of the GABAA receptor channel of mouse spinal neurones in culture. J Physiol. 1989 Oct;417:483–500. doi: 10.1113/jphysiol.1989.sp017814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathers D. A., Barker J. L. (-)Pentobarbital opens ion channels of long duration in cultured mouse spinal neurons. Science. 1980 Jul 25;209(4455):507–509. doi: 10.1126/science.6248961. [DOI] [PubMed] [Google Scholar]
- Nicoll R. A., Wojtowicz J. M. The effects of pentobarbital and related compounds on frog motoneurons. Brain Res. 1980 Jun 2;191(1):225–237. doi: 10.1016/0006-8993(80)90325-x. [DOI] [PubMed] [Google Scholar]
- Okonjo K. O., Kuhlmann J., Maelicke A. A second pathway of activation of the Torpedo acetylcholine receptor channel. Eur J Biochem. 1991 Sep 15;200(3):671–677. doi: 10.1111/j.1432-1033.1991.tb16231.x. [DOI] [PubMed] [Google Scholar]
- Olsen R. W. Barbiturates. Int Anesthesiol Clin. 1988 Winter;26(4):254–261. doi: 10.1097/00004311-198802640-00004. [DOI] [PubMed] [Google Scholar]
- Orser B. A., Wang L. Y., Pennefather P. S., MacDonald J. F. Propofol modulates activation and desensitization of GABAA receptors in cultured murine hippocampal neurons. J Neurosci. 1994 Dec;14(12):7747–7760. doi: 10.1523/JNEUROSCI.14-12-07747.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker I., Gundersen C. B., Miledi R. Actions of pentobarbital on rat brain receptors expressed in Xenopus oocytes. J Neurosci. 1986 Aug;6(8):2290–2297. doi: 10.1523/JNEUROSCI.06-08-02290.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J. A., Kirkness E. F., Callachan H., Lambert J. J., Turner A. J. Modulation of the GABAA receptor by depressant barbiturates and pregnane steroids. Br J Pharmacol. 1988 Aug;94(4):1257–1269. doi: 10.1111/j.1476-5381.1988.tb11646.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson B. Actions of anaesthetics and avermectin on GABAA chloride channels in mammalian dorsal root ganglion neurones. Br J Pharmacol. 1989 Sep;98(1):167–176. doi: 10.1111/j.1476-5381.1989.tb16878.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers C. J., Twyman R. E., Macdonald R. L. Benzodiazepine and beta-carboline regulation of single GABAA receptor channels of mouse spinal neurones in culture. J Physiol. 1994 Feb 15;475(1):69–82. doi: 10.1113/jphysiol.1994.sp020050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanna E., Garau F., Harris R. A. Novel properties of homomeric beta 1 gamma-aminobutyric acid type A receptors: actions of the anesthetics propofol and pentobarbital. Mol Pharmacol. 1995 Feb;47(2):213–217. [PubMed] [Google Scholar]
- Schofield P. R., Darlison M. G., Fujita N., Burt D. R., Stephenson F. A., Rodriguez H., Rhee L. M., Ramachandran J., Reale V., Glencorse T. A. Sequence and functional expression of the GABA A receptor shows a ligand-gated receptor super-family. Nature. 1987 Jul 16;328(6127):221–227. doi: 10.1038/328221a0. [DOI] [PubMed] [Google Scholar]
- Schulz D. W., Macdonald R. L. Barbiturate enhancement of GABA-mediated inhibition and activation of chloride ion conductance: correlation with anticonvulsant and anesthetic actions. Brain Res. 1981 Mar 23;209(1):177–188. doi: 10.1016/0006-8993(81)91179-3. [DOI] [PubMed] [Google Scholar]
- Schwartz R. D., Suzdak P. D., Paul S. M. gamma-Aminobutyric acid (GABA)- and barbiturate-mediated 36Cl- uptake in rat brain synaptoneurosomes: evidence for rapid desensitization of the GABA receptor-coupled chloride ion channel. Mol Pharmacol. 1986 Nov;30(5):419–426. [PubMed] [Google Scholar]
- Sigel E., Baur R., Trube G., Möhler H., Malherbe P. The effect of subunit composition of rat brain GABAA receptors on channel function. Neuron. 1990 Nov;5(5):703–711. doi: 10.1016/0896-6273(90)90224-4. [DOI] [PubMed] [Google Scholar]
- Smith G. B., Olsen R. W. Functional domains of GABAA receptors. Trends Pharmacol Sci. 1995 May;16(5):162–168. doi: 10.1016/s0165-6147(00)89009-4. [DOI] [PubMed] [Google Scholar]
- Study R. E., Barker J. L. Diazepam and (--)-pentobarbital: fluctuation analysis reveals different mechanisms for potentiation of gamma-aminobutyric acid responses in cultured central neurons. Proc Natl Acad Sci U S A. 1981 Nov;78(11):7180–7184. doi: 10.1073/pnas.78.11.7180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanelian D. L., Kosek P., Mody I., MacIver M. B. The role of the GABAA receptor/chloride channel complex in anesthesia. Anesthesiology. 1993 Apr;78(4):757–776. doi: 10.1097/00000542-199304000-00020. [DOI] [PubMed] [Google Scholar]
- Wafford K. A., Bain C. J., Quirk K., McKernan R. M., Wingrove P. B., Whiting P. J., Kemp J. A. A novel allosteric modulatory site on the GABAA receptor beta subunit. Neuron. 1994 Apr;12(4):775–782. doi: 10.1016/0896-6273(94)90330-1. [DOI] [PubMed] [Google Scholar]
- Wafford K. A., Bain C. J., Whiting P. J., Kemp J. A. Functional comparison of the role of gamma subunits in recombinant human gamma-aminobutyric acidA/benzodiazepine receptors. Mol Pharmacol. 1993 Aug;44(2):437–442. [PubMed] [Google Scholar]
- Wafford K. A., Whiting P. J., Kemp J. A. Differences in affinity and efficacy of benzodiazepine receptor ligands at recombinant gamma-aminobutyric acidA receptor subtypes. Mol Pharmacol. 1993 Feb;43(2):240–244. [PubMed] [Google Scholar]
- Wakamori M., Ikemoto Y., Akaike N. Effects of two volatile anesthetics and a volatile convulsant on the excitatory and inhibitory amino acid responses in dissociated CNS neurons of the rat. J Neurophysiol. 1991 Dec;66(6):2014–2021. doi: 10.1152/jn.1991.66.6.2014. [DOI] [PubMed] [Google Scholar]
- Wisden W., Laurie D. J., Monyer H., Seeburg P. H. The distribution of 13 GABAA receptor subunit mRNAs in the rat brain. I. Telencephalon, diencephalon, mesencephalon. J Neurosci. 1992 Mar;12(3):1040–1062. doi: 10.1523/JNEUROSCI.12-03-01040.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman S. A., Jones M. V., Harrison N. L. Potentiation of gamma-aminobutyric acidA receptor Cl- current correlates with in vivo anesthetic potency. J Pharmacol Exp Ther. 1994 Sep;270(3):987–991. [PubMed] [Google Scholar]



