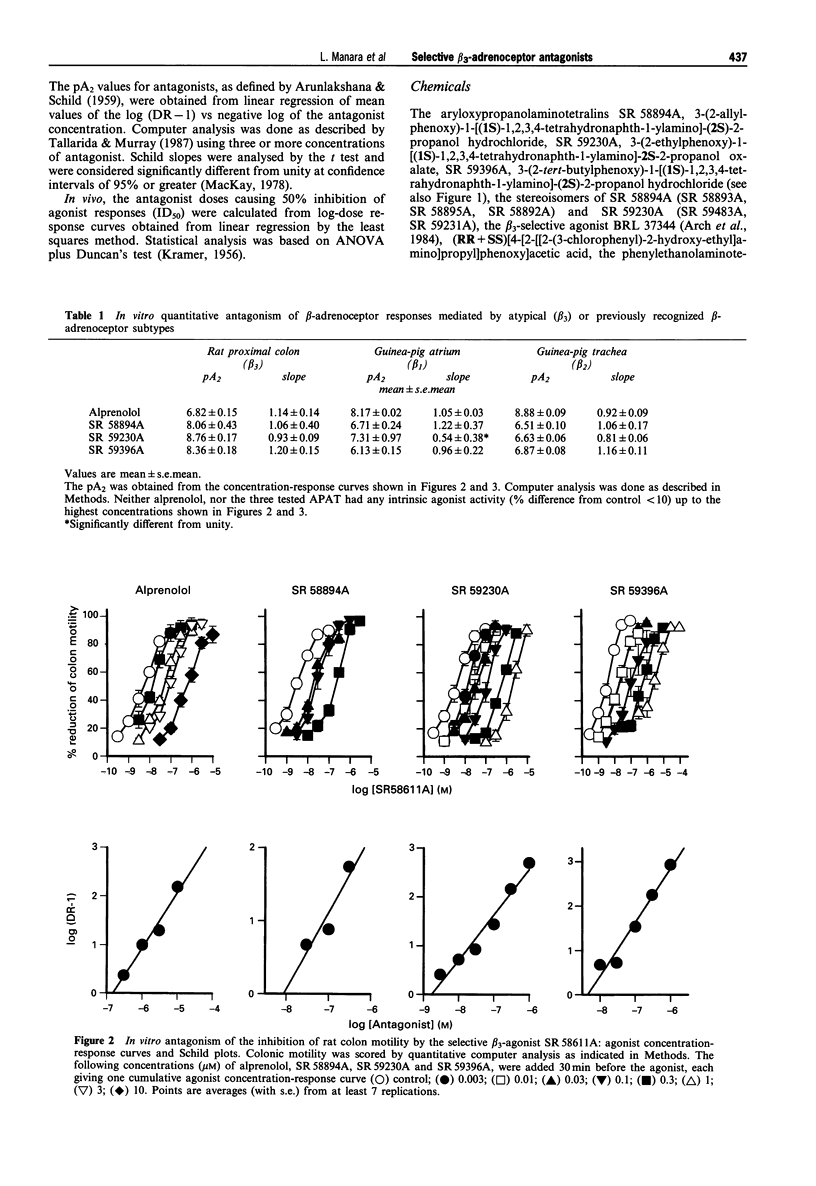
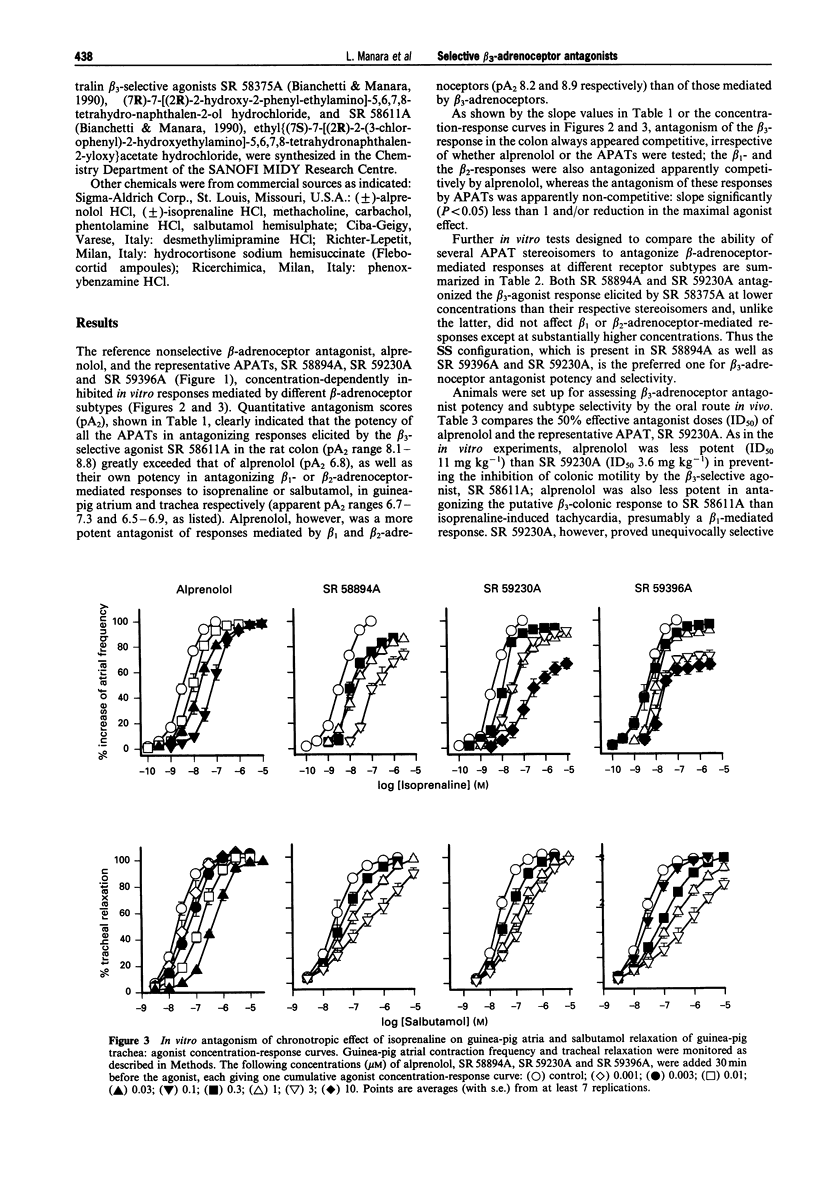
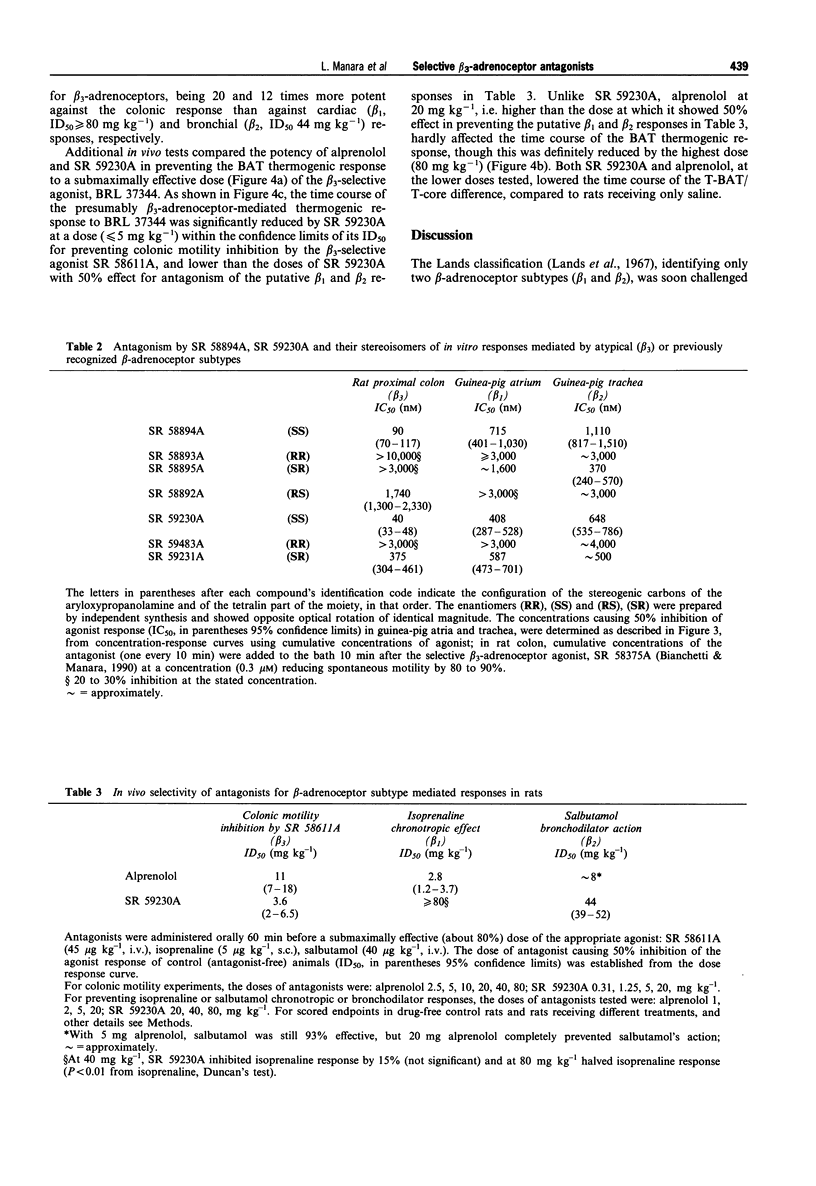
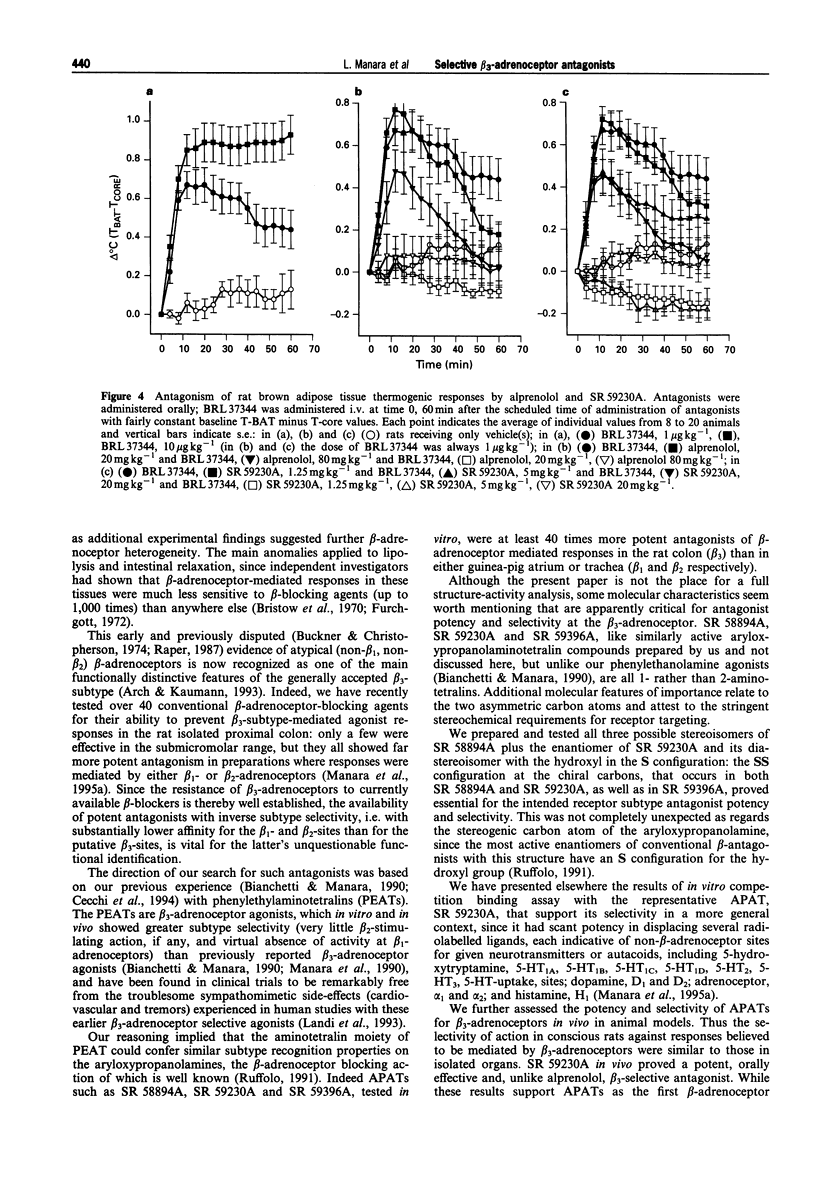
Abstract
1. We have assessed the relative abilities of compounds belonging to the new aryloxypropanolaminotetralin (APAT) class and of the reference beta-adrenoceptor-blocking agent, alprenolol, to antagonize functional responses in vitro and in vivo involving atypical (beta 3) or conventional (beta 1 and beta 2) beta-adrenoceptors. 2. The range of pA2 values for three representative APATs against inhibition of spontaneous motility in the rat isolated colon by the selective beta 3-adrenoceptor agonist, SR 58611A (8.1-8.8), was well above similarly calculated values for non-competitive antagonism of guinea-pig trachea relaxation by salbutamol (beta 2, 6.5-6.9) and the atrial chronotropic response by isoprenaline (beta 1, 6.7-7.3). Alprenolol, however, was substantially more potent in antagonizing atrial (pA2, 8.2) and tracheal (pA2, 8.9) responses than SR 58611A mediated inhibition of colonic motility (pA2, 6.8). 3. Several APAT isomers with different configurations at the chiral carbons, when tested on isolated organs, presented stringent stereochemical requirements for beta 3-selectivity, including high antagonist potency-ratios between active and inactive enantiomers. 4. In vivo, the inhibition of colonic motility and the thermogenic response of brown adipose tissue elicited in rats by the selective beta 3-adrenoceptor agonists SR 58611A and BRL 37344 respectively were substantially diminished by the representative APAT, SR 59230A, at oral doses (< or = 5 mg kg-1) well below those half maximally effective (ID50) for preventing beta 1-(isoprenaline tachycardia > or = 80 mg kg-1) or beta 2-(salbutamol bronchodilatation, 44 mg kg-1) mediated responses. Alprenolol, as expected, was a less potent and nonselective antagonist of the putative beta 3-responses. 5. These findings support APATs as the first potent, orally effective selective antagonists at beta 3-adrenoceptors, and provide final unambiguous evidence that beta 3-adrenoceptors underlie inhibition of colonic motility and brown adipose tissue thermogenesis in rats.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARUNLAKSHANA O., SCHILD H. O. Some quantitative uses of drug antagonists. Br J Pharmacol Chemother. 1959 Mar;14(1):48–58. doi: 10.1111/j.1476-5381.1959.tb00928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arch J. R., Ainsworth A. T., Cawthorne M. A., Piercy V., Sennitt M. V., Thody V. E., Wilson C., Wilson S. Atypical beta-adrenoceptor on brown adipocytes as target for anti-obesity drugs. Nature. 1984 May 10;309(5964):163–165. doi: 10.1038/309163a0. [DOI] [PubMed] [Google Scholar]
- Arch J. R., Kaumann A. J. Beta 3 and atypical beta-adrenoceptors. Med Res Rev. 1993 Nov;13(6):663–729. doi: 10.1002/med.2610130604. [DOI] [PubMed] [Google Scholar]
- Bensaid M., Kaghad M., Rodriguez M., Le Fur G., Caput D. The rat beta 3-adrenergic receptor gene contains an intron. FEBS Lett. 1993 Mar 8;318(3):223–226. doi: 10.1016/0014-5793(93)80516-w. [DOI] [PubMed] [Google Scholar]
- Bianchetti A., Manara L. In vitro inhibition of intestinal motility by phenylethanolaminotetralines: evidence of atypical beta-adrenoceptors in rat colon. Br J Pharmacol. 1990 Aug;100(4):831–839. doi: 10.1111/j.1476-5381.1990.tb14100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bristow M., Sherrod T. R., Green R. D. Analysis of beta receptor drug interactions in isolated rabbit atrium, aorta, stomach and trachea. J Pharmacol Exp Ther. 1970 Jan;171(1):52–61. [PubMed] [Google Scholar]
- Buckner C. K., Christopherson R. C. Adrenergic receptors of rat esophageal smooth muscle. J Pharmacol Exp Ther. 1974 May;189(2):467–475. [PubMed] [Google Scholar]
- Emorine L. J., Marullo S., Briend-Sutren M. M., Patey G., Tate K., Delavier-Klutchko C., Strosberg A. D. Molecular characterization of the human beta 3-adrenergic receptor. Science. 1989 Sep 8;245(4922):1118–1121. doi: 10.1126/science.2570461. [DOI] [PubMed] [Google Scholar]
- Granneman J. G., Lahners K. N., Chaudhry A. Molecular cloning and expression of the rat beta 3-adrenergic receptor. Mol Pharmacol. 1991 Dec;40(6):895–899. [PubMed] [Google Scholar]
- Lands A. M., Arnold A., McAuliff J. P., Luduena F. P., Brown T. G., Jr Differentiation of receptor systems activated by sympathomimetic amines. Nature. 1967 May 6;214(5088):597–598. doi: 10.1038/214597a0. [DOI] [PubMed] [Google Scholar]
- Lelias J. M., Kaghad M., Rodriguez M., Chalon P., Bonnin J., Dupre I., Delpech B., Bensaid M., LeFur G., Ferrara P. Molecular cloning of a human beta 3-adrenergic receptor cDNA. FEBS Lett. 1993 Jun 14;324(2):127–130. doi: 10.1016/0014-5793(93)81377-c. [DOI] [PubMed] [Google Scholar]
- MacKay D. How should values of pA2 and affinity constants for pharmacological competitive antagonists be estimated? J Pharm Pharmacol. 1978 May;30(5):312–313. doi: 10.1111/j.2042-7158.1978.tb13237.x. [DOI] [PubMed] [Google Scholar]
- Manara L., Croci T., Landi M. Beta 3-adrenoceptors and intestinal motility. Fundam Clin Pharmacol. 1995;9(4):332–342. doi: 10.1111/j.1472-8206.1995.tb00507.x. [DOI] [PubMed] [Google Scholar]
- Muzzin P., Revelli J. P., Kuhne F., Gocayne J. D., McCombie W. R., Venter J. C., Giacobino J. P., Fraser C. M. An adipose tissue-specific beta-adrenergic receptor. Molecular cloning and down-regulation in obesity. J Biol Chem. 1991 Dec 15;266(35):24053–24058. [PubMed] [Google Scholar]
- POWELL C. E., SLATER I. H. Blocking of inhibitory adrenergic receptors by a dichloro analog of isoproterenol. J Pharmacol Exp Ther. 1958 Apr;122(4):480–488. [PubMed] [Google Scholar]
- Pennock B. E., Cox C. P., Rogers R. M., Cain W. A., Wells J. H. A noninvasive technique for measurement of changes in specific airway resistance. J Appl Physiol Respir Environ Exerc Physiol. 1979 Feb;46(2):399–406. doi: 10.1152/jappl.1979.46.2.399. [DOI] [PubMed] [Google Scholar]
- Raper C. Adrenoceptor classification. Clin Exp Pharmacol Physiol. 1987 May;14(5):401–407. doi: 10.1111/j.1440-1681.1987.tb00990.x. [DOI] [PubMed] [Google Scholar]
- Ratkowsky D. A., Reedy T. J. Choosing near-linear parameters in the four-parameter logistic model for radioligand and related assays. Biometrics. 1986 Sep;42(3):575–582. [PubMed] [Google Scholar]
- Zaagsma J., Nahorski S. R. Is the adipocyte beta-adrenoceptor a prototype for the recently cloned atypical 'beta 3-adrenoceptor'? Trends Pharmacol Sci. 1990 Jan;11(1):3–7. doi: 10.1016/0165-6147(90)90032-4. [DOI] [PubMed] [Google Scholar]


