Abstract
1 The angiogenic activity of four vasoactive peptides with a range of vasodilator and vasoconstrictor properties, i.e. vasoactive intestinal peptide (VIP), endothelin-1, endothelin-3 and angiotensin II, were investigated in a rat sponge model. Neovascularization was assessed by the 133Xe clearance technique and confirmed by histological studies. 2 Daily doses of the vasodilator peptide, VIP (1000 pmol), caused intense neovascularization, but a lower dose (10 pmol) produced no apparent effect. However, the lower dose of VIP, when given with a subthreshold dose of interleukin-1 alpha (0.3 pmol), produced an angiogenic response similar to that seen with the higher dose of VIP. The neovascular response induced by co-administration of VIP and interleukin-1 alpha was inhibited by simultaneous administration of 100 pmol VIP (10-28), a specific VIP receptor antagonist. 3 In contrast, daily doses of 10, 100 or 1000 pmol endothelin-3 (a mixed vasoconstrictor and vasodilator with more marked vasodilator activity) or of 100 or 1000 pmol endothelin-1 (also with mixed activity but with much more pronounced vasoconstrictor response) produced no apparent effect on sponge-induced angiogenesis. 4 The vasoconstrictor peptide, angiotensin II, in daily doses of 1000 pmol, caused an intense neovascularization like VIP but lower doses of angiotensin II (10 or 100 pmol) produced no apparent effect. The lowest dose of angiotensin II (10 pmol) when administered with the subthreshold dose of interleukin-1 alpha (0.3 pmol) had no effect on the basal neovascular response in the sponges. The angiotensin II-induced neovascular response was inhibited by co-administration of 100 nmol of the specific AT1 receptor antagonist, losartan, but not by the AT2 receptor antagonist, PD 123319. 5 These data show that VIP and angiotensin II possess angiogenic activity. However, endothelin-1 and endothelin-3 had no activity at the doses used. Thus the angiogenic response is not related to local vasoconstriction or vasodilatation in the sponges. The blockade of VIP- and angiotensin II-induced angiogenesis at the receptor level suggests that receptor modulation could provide a strategy for the management of angiogenic diseases.
Full text
PDF

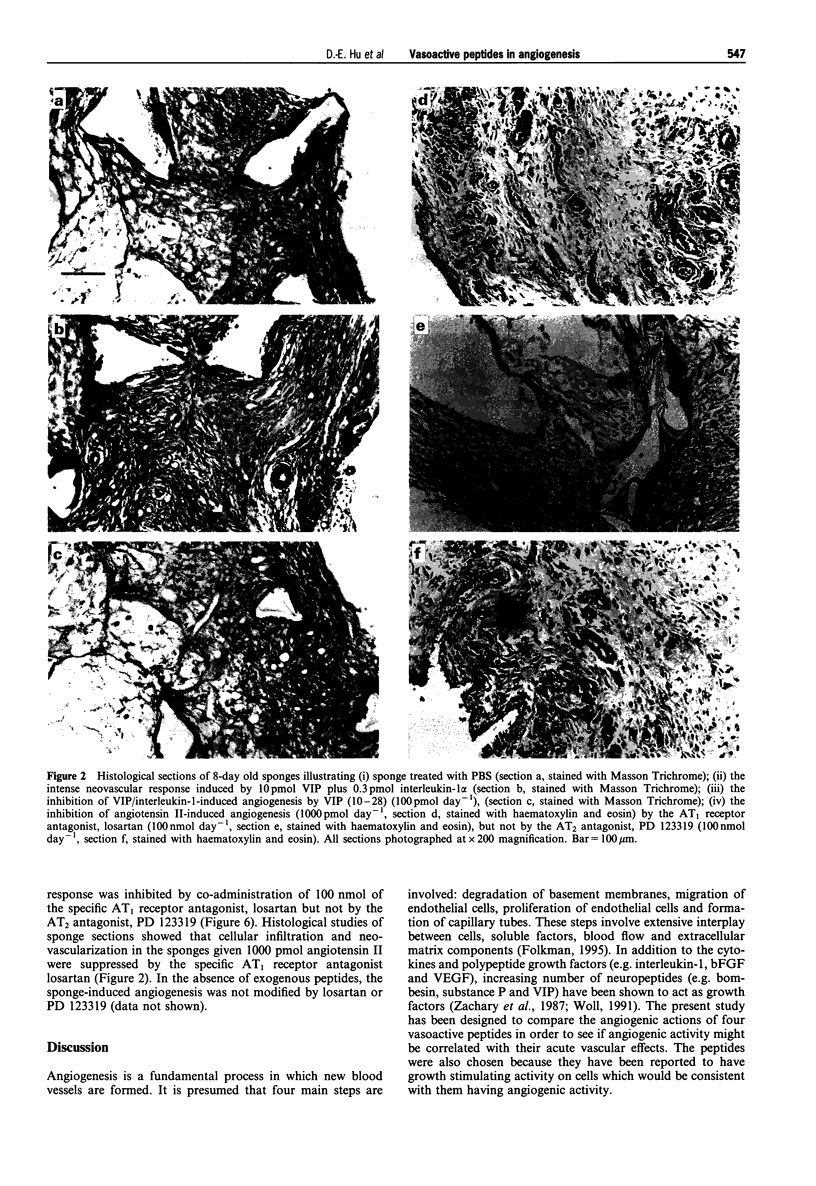
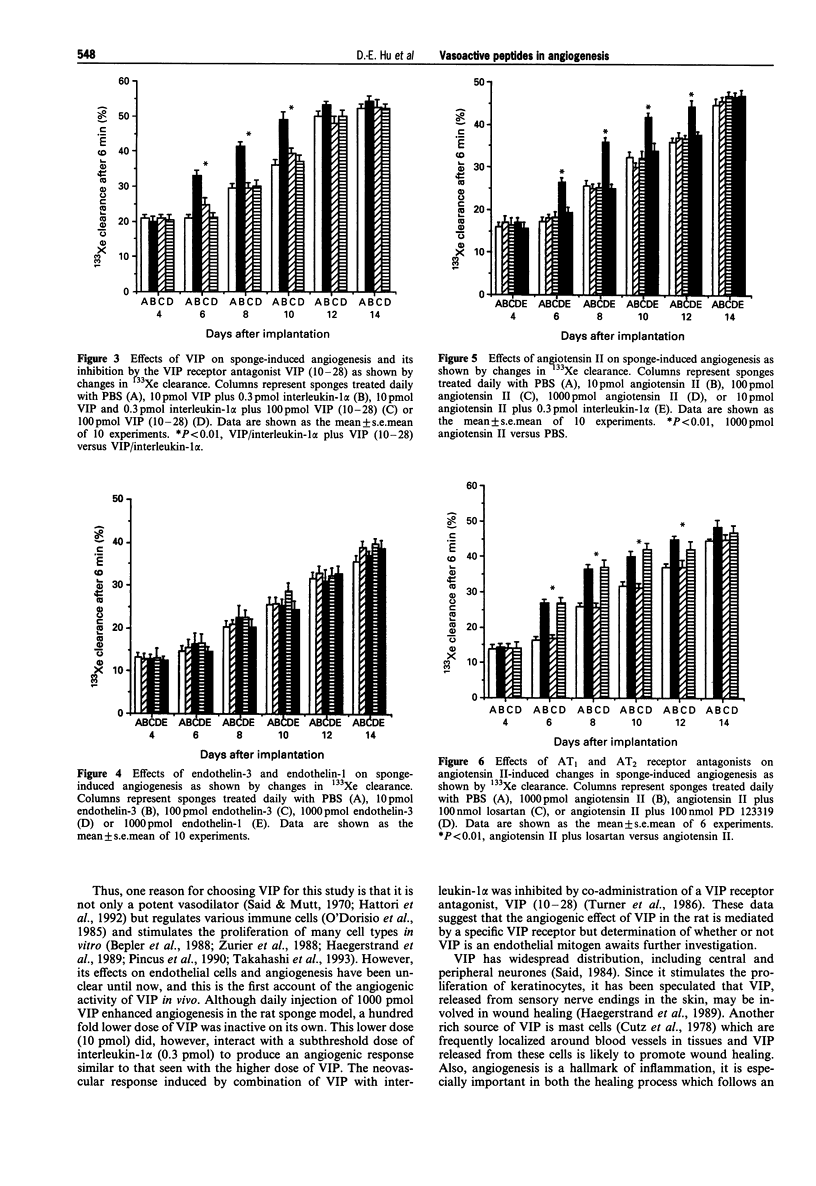



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrade S. P., Fan T. P., Lewis G. P. Quantitative in-vivo studies on angiogenesis in a rat sponge model. Br J Exp Pathol. 1987 Dec;68(6):755–766. [PMC free article] [PubMed] [Google Scholar]
- Arai K. I., Lee F., Miyajima A., Miyatake S., Arai N., Yokota T. Cytokines: coordinators of immune and inflammatory responses. Annu Rev Biochem. 1990;59:783–836. doi: 10.1146/annurev.bi.59.070190.004031. [DOI] [PubMed] [Google Scholar]
- Bepler G., Rotsch M., Jaques G., Haeder M., Heymanns J., Hartogh G., Kiefer P., Havemann K. Peptides and growth factors in small cell lung cancer: production, binding sites, and growth effects. J Cancer Res Clin Oncol. 1988;114(3):235–244. doi: 10.1007/BF00405828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley T. L., Brain S. D., Collins P. D., Williams T. J. Inflammatory edema induced by interactions between IL-1 and the neuropeptide calcitonin gene-related peptide. J Immunol. 1991 May 15;146(10):3424–3430. [PubMed] [Google Scholar]
- Carlini R. G., Dusso A. S., Obialo C. I., Alvarez U. M., Rothstein M. Recombinant human erythropoietin (rHuEPO) increases endothelin-1 release by endothelial cells. Kidney Int. 1993 May;43(5):1010–1014. doi: 10.1038/ki.1993.142. [DOI] [PubMed] [Google Scholar]
- Carlini R. G., Reyes A. A., Rothstein M. Recombinant human erythropoietin stimulates angiogenesis in vitro. Kidney Int. 1995 Mar;47(3):740–745. doi: 10.1038/ki.1995.113. [DOI] [PubMed] [Google Scholar]
- Cutz E., Chan W., Track N. S., Goth A., Said S. I. Release of vasoactive intestinal polypeptide in mast cells by histamine liberators. Nature. 1978 Oct 19;275(5681):661–662. doi: 10.1038/275661a0. [DOI] [PubMed] [Google Scholar]
- Dashwood M. R., Allen S. P., Luu T. N., Muddle J. R. The effect of the ETA receptor antagonist, FR 139317, on [125I]-ET-1 binding to the atherosclerotic human coronary artery. Br J Pharmacol. 1994 Jun;112(2):386–389. doi: 10.1111/j.1476-5381.1994.tb13083.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello C. A. Interleukin-1 and interleukin-1 antagonism. Blood. 1991 Apr 15;77(8):1627–1652. [PubMed] [Google Scholar]
- Douglas S. A., Hiley C. R. Endothelium-dependent mesenteric vasorelaxant effects and systemic actions of endothelin (16-21) and other endothelin-related peptides in the rat. Br J Pharmacol. 1991 Oct;104(2):311–320. doi: 10.1111/j.1476-5381.1991.tb12428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas S. A., Hiley C. R. Endothelium-dependent vascular activities of endothelin-like peptides in the isolated superior mesenteric arterial bed of the rat. Br J Pharmacol. 1990 Sep;101(1):81–88. doi: 10.1111/j.1476-5381.1990.tb12093.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Economos K., MacDonald P. C., Casey M. L. Endothelin-1 gene expression and biosynthesis in human endometrial HEC-1A cancer cells. Cancer Res. 1992 Feb 1;52(3):554–557. [PubMed] [Google Scholar]
- Estival A., Mouniélou P., Trocheris V., Scemama J. L., Clemente F., Hollande E., Ribet A. Presence of VIP receptors in a human pancreatic adenocarcinoma cell line. Modulation of the cAMP response during cell proliferation. Biochem Biophys Res Commun. 1983 Mar 29;111(3):958–963. doi: 10.1016/0006-291x(83)91393-1. [DOI] [PubMed] [Google Scholar]
- Fan T. P., Hu D. E., Guard S., Gresham G. A., Watling K. J. Stimulation of angiogenesis by substance P and interleukin-1 in the rat and its inhibition by NK1 or interleukin-1 receptor antagonists. Br J Pharmacol. 1993 Sep;110(1):43–49. doi: 10.1111/j.1476-5381.1993.tb13769.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan T. P., Jaggar R., Bicknell R. Controlling the vasculature: angiogenesis, anti-angiogenesis and vascular targeting of gene therapy. Trends Pharmacol Sci. 1995 Feb;16(2):57–66. doi: 10.1016/s0165-6147(00)88979-8. [DOI] [PubMed] [Google Scholar]
- Fernandez L. A., Caride V. J., Twickler J., Galardy R. E. Renin-angiotensin and development of collateral circulation after renal ischemia. Am J Physiol. 1982 Dec;243(6):H869–H875. doi: 10.1152/ajpheart.1982.243.6.H869. [DOI] [PubMed] [Google Scholar]
- Fernandez L. A., Twickler J., Mead A. Neovascularization produced by angiotensin II. J Lab Clin Med. 1985 Feb;105(2):141–145. [PubMed] [Google Scholar]
- Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995 Jan;1(1):27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- Folkman J., Shing Y. Angiogenesis. J Biol Chem. 1992 Jun 5;267(16):10931–10934. [PubMed] [Google Scholar]
- Haegerstrand A., Jonzon B., Dalsgaard C. J., Nilsson J. Vasoactive intestinal polypeptide stimulates cell proliferation and adenylate cyclase activity of cultured human keratinocytes. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5993–5996. doi: 10.1073/pnas.86.15.5993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn A. W., Resink T. J., Kern F., Bühler F. R. Peptide vasoconstrictors, vessel structure, and vascular smooth-muscle proliferation. J Cardiovasc Pharmacol. 1993;22 (Suppl 5):S37–S43. doi: 10.1097/00005344-199322005-00007. [DOI] [PubMed] [Google Scholar]
- Hattori Y., Nagashima M., Endo Y., Kanno M. Glibenclamide does not block arterial relaxation caused by vasoactive intestinal polypeptide. Eur J Pharmacol. 1992 Mar 17;213(1):147–150. doi: 10.1016/0014-2999(92)90246-z. [DOI] [PubMed] [Google Scholar]
- Hirata Y., Takagi Y., Fukuda Y., Marumo F. Endothelin is a potent mitogen for rat vascular smooth muscle cells. Atherosclerosis. 1989 Aug;78(2-3):225–228. doi: 10.1016/0021-9150(89)90227-x. [DOI] [PubMed] [Google Scholar]
- Hu D. E., Fan T. P. Protein kinase C inhibitor calphostin C prevents cytokine-induced angiogenesis in the rat. Inflammation. 1995 Feb;19(1):39–54. doi: 10.1007/BF01534379. [DOI] [PubMed] [Google Scholar]
- Hu D. E., Fan T. P. Suppression of VEGF-induced angiogenesis by the protein tyrosine kinase inhibitor, lavendustin A. Br J Pharmacol. 1995 Jan;114(2):262–268. doi: 10.1111/j.1476-5381.1995.tb13221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu D. E., Fan T. P. [Leu8]des-Arg9-bradykinin inhibits the angiogenic effect of bradykinin and interleukin-1 in rats. Br J Pharmacol. 1993 May;109(1):14–17. doi: 10.1111/j.1476-5381.1993.tb13525.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu D. E., Hiley C. R., Smither R. L., Gresham G. A., Fan T. P. Correlation of 133Xe clearance, blood flow and histology in the rat sponge model for angiogenesis. Further studies with angiogenic modifiers. Lab Invest. 1995 May;72(5):601–610. [PubMed] [Google Scholar]
- Hu D. E., Hori Y., Fan T. P. Interleukin-8 stimulates angiogenesis in rats. Inflammation. 1993 Apr;17(2):135–143. doi: 10.1007/BF00916100. [DOI] [PubMed] [Google Scholar]
- Hu D. E., Hori Y., Presta M., Gresham G. A., Fan T. P. Inhibition of angiogenesis in rats by IL-1 receptor antagonist and selected cytokine antibodies. Inflammation. 1994 Feb;18(1):45–58. doi: 10.1007/BF01534597. [DOI] [PubMed] [Google Scholar]
- Kimura B., Sumners C., Phillips M. I. Changes in skin angiotensin II receptors in rats during wound healing. Biochem Biophys Res Commun. 1992 Sep 16;187(2):1083–1090. doi: 10.1016/0006-291x(92)91308-d. [DOI] [PubMed] [Google Scholar]
- Komuro I., Kurihara H., Sugiyama T., Yoshizumi M., Takaku F., Yazaki Y. Endothelin stimulates c-fos and c-myc expression and proliferation of vascular smooth muscle cells. FEBS Lett. 1988 Oct 10;238(2):249–252. doi: 10.1016/0014-5793(88)80489-7. [DOI] [PubMed] [Google Scholar]
- Krämer B. K., Nishida M., Kelly R. A., Smith T. W. Endothelins. Myocardial actions of a new class of cytokines. Circulation. 1992 Jan;85(1):350–356. doi: 10.1161/01.cir.85.1.350. [DOI] [PubMed] [Google Scholar]
- Le Noble F. A., Schreurs N. H., van Straaten H. W., Slaaf D. W., Smits J. F., Rogg H., Struijker-Boudier H. A. Evidence for a novel angiotensin II receptor involved in angiogenesis in chick embryo chorioallantoic membrane. Am J Physiol. 1993 Feb;264(2 Pt 2):R460–R465. doi: 10.1152/ajpregu.1993.264.2.R460. [DOI] [PubMed] [Google Scholar]
- Moghaddam A., Zhang H. T., Fan T. P., Hu D. E., Lees V. C., Turley H., Fox S. B., Gatter K. C., Harris A. L., Bicknell R. Thymidine phosphorylase is angiogenic and promotes tumor growth. Proc Natl Acad Sci U S A. 1995 Feb 14;92(4):998–1002. doi: 10.1073/pnas.92.4.998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muegge K., Durum S. K. Cytokines and transcription factors. Cytokine. 1990 Jan;2(1):1–8. doi: 10.1016/1043-4666(90)90036-s. [DOI] [PubMed] [Google Scholar]
- O'Dorisio M. S., Wood C. L., O'Dorisio T. M. Vasoactive intestinal peptide and neuropeptide modulation of the immune response. J Immunol. 1985 Aug;135(2 Suppl):792s–796s. [PubMed] [Google Scholar]
- Pincus D. W., DiCicco-Bloom E. M., Black I. B. Vasoactive intestinal peptide regulates mitosis, differentiation and survival of cultured sympathetic neuroblasts. Nature. 1990 Feb 8;343(6258):564–567. doi: 10.1038/343564a0. [DOI] [PubMed] [Google Scholar]
- Sadoshima J., Izumo S. Molecular characterization of angiotensin II--induced hypertrophy of cardiac myocytes and hyperplasia of cardiac fibroblasts. Critical role of the AT1 receptor subtype. Circ Res. 1993 Sep;73(3):413–423. doi: 10.1161/01.res.73.3.413. [DOI] [PubMed] [Google Scholar]
- Said S. I., Faloona G. R. Elevated plasma and tissue levels of vasoactive intestinal polypeptide in the watery-diarrhea syndrome due to pancreatic, bronchogenic and other tumors. N Engl J Med. 1975 Jul 24;293(4):155–160. doi: 10.1056/NEJM197507242930401. [DOI] [PubMed] [Google Scholar]
- Said S. I., Mutt V. Potent peripheral and splanchnic vasodilator peptide from normal gut. Nature. 1970 Feb 28;225(5235):863–864. doi: 10.1038/225863a0. [DOI] [PubMed] [Google Scholar]
- Said S. I. Vasoactive intestinal polypeptide (VIP): current status. Peptides. 1984 Mar-Apr;5(2):143–150. doi: 10.1016/0196-9781(84)90197-9. [DOI] [PubMed] [Google Scholar]
- Saklatvala J., Rawlinson L. M., Marshall C. J., Kracht M. Interleukin 1 and tumour necrosis factor activate the mitogen-activated protein (MAP) kinase kinase in cultured cells. FEBS Lett. 1993 Nov 15;334(2):189–192. doi: 10.1016/0014-5793(93)81709-9. [DOI] [PubMed] [Google Scholar]
- Serradeil-Le Gal C., Herbert J. M., Garcia C., Boutin M., Maffrand J. P. Importance of the phenotypic state of vascular smooth muscle cells on the binding and the mitogenic activity of endothelin. Peptides. 1991 May-Jun;12(3):575–579. doi: 10.1016/0196-9781(91)90104-w. [DOI] [PubMed] [Google Scholar]
- Stoll M., Steckelings U. M., Paul M., Bottari S. P., Metzger R., Unger T. The angiotensin AT2-receptor mediates inhibition of cell proliferation in coronary endothelial cells. J Clin Invest. 1995 Feb;95(2):651–657. doi: 10.1172/JCI117710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K., Nakanishi S., Imamura S. Direct effects of cutaneous neuropeptides on adenylyl cyclase activity and proliferation in a keratinocyte cell line: stimulation of cyclic AMP formation by CGRP and VIP/PHM, and inhibition by NPY through G protein-coupled receptors. J Invest Dermatol. 1993 Nov;101(5):646–651. doi: 10.1111/1523-1747.ep12371670. [DOI] [PubMed] [Google Scholar]
- Timmermans P. B., Wong P. C., Chiu A. T., Herblin W. F., Benfield P., Carini D. J., Lee R. J., Wexler R. R., Saye J. A., Smith R. D. Angiotensin II receptors and angiotensin II receptor antagonists. Pharmacol Rev. 1993 Jun;45(2):205–251. [PubMed] [Google Scholar]
- Tsuda T., Kawahara Y., Shii K., Koide M., Ishida Y., Yokoyama M. Vasoconstrictor-induced protein-tyrosine phosphorylation in cultured vascular smooth muscle cells. FEBS Lett. 1991 Jul 8;285(1):44–48. doi: 10.1016/0014-5793(91)80721-e. [DOI] [PubMed] [Google Scholar]
- Turner J. T., Jones S. B., Bylund D. B. A fragment of vasoactive intestinal peptide, VIP(10-28), is an antagonist of VIP in the colon carcinoma cell line, HT29. Peptides. 1986 Sep-Oct;7(5):849–854. doi: 10.1016/0196-9781(86)90105-1. [DOI] [PubMed] [Google Scholar]
- Vigne P., Marsault R., Breittmayer J. P., Frelin C. Endothelin stimulates phosphatidylinositol hydrolysis and DNA synthesis in brain capillary endothelial cells. Biochem J. 1990 Mar 1;266(2):415–420. doi: 10.1042/bj2660415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virgolini I., Raderer M., Kurtaran A., Angelberger P., Banyai S., Yang Q., Li S., Banyai M., Pidlich J., Niederle B. Vasoactive intestinal peptide-receptor imaging for the localization of intestinal adenocarcinomas and endocrine tumors. N Engl J Med. 1994 Oct 27;331(17):1116–1121. doi: 10.1056/NEJM199410273311703. [DOI] [PubMed] [Google Scholar]
- Warner T. D., de Nucci G., Vane J. R. Rat endothelin is a vasodilator in the isolated perfused mesentery of the rat. Eur J Pharmacol. 1989 Jan 17;159(3):325–326. doi: 10.1016/0014-2999(89)90167-2. [DOI] [PubMed] [Google Scholar]
- Waschek J. A. Vasoactive intestinal peptide: an important trophic factor and developmental regulator? Dev Neurosci. 1995;17(1):1–7. doi: 10.1159/000111268. [DOI] [PubMed] [Google Scholar]
- Weber H., Taylor D. S., Molloy C. J. Angiotensin II induces delayed mitogenesis and cellular proliferation in rat aortic smooth muscle cells. Correlation with the expression of specific endogenous growth factors and reversal by suramin. J Clin Invest. 1994 Feb;93(2):788–798. doi: 10.1172/JCI117033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber H., Webb M. L., Serafino R., Taylor D. S., Moreland S., Norman J., Molloy C. J. Endothelin-1 and angiotensin-II stimulate delayed mitogenesis in cultured rat aortic smooth muscle cells: evidence for common signaling mechanisms. Mol Endocrinol. 1994 Feb;8(2):148–158. doi: 10.1210/mend.8.2.8170471. [DOI] [PubMed] [Google Scholar]
- Woll P. J. Neuropeptide growth factors and cancer. Br J Cancer. 1991 Mar;63(3):469–475. doi: 10.1038/bjc.1991.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wren A. D., Hiley C. R., Fan T. P. Endothelin-3 mediated proliferation in wounded human umbilical vein endothelial cells. Biochem Biophys Res Commun. 1993 Oct 15;196(1):369–375. doi: 10.1006/bbrc.1993.2258. [DOI] [PubMed] [Google Scholar]
- Wright C. E., Fozard J. R. Regional vasodilation is a prominent feature of the haemodynamic response to endothelin in anaesthetized, spontaneously hypertensive rats. Eur J Pharmacol. 1988 Oct 11;155(1-2):201–203. doi: 10.1016/0014-2999(88)90425-6. [DOI] [PubMed] [Google Scholar]
- Yanagisawa M., Kurihara H., Kimura S., Tomobe Y., Kobayashi M., Mitsui Y., Yazaki Y., Goto K., Masaki T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988 Mar 31;332(6163):411–415. doi: 10.1038/332411a0. [DOI] [PubMed] [Google Scholar]
- Yu S. T., Yu C. C., Wong H. W., Yuan H. T. Effect of endothelin-1 on the vascular smooth muscle cell cycle. J Cardiovasc Pharmacol. 1991;17 (Suppl 7):S239–S241. doi: 10.1097/00005344-199100177-00069. [DOI] [PubMed] [Google Scholar]
- Zachary I., Woll P. J., Rozengurt E. A role for neuropeptides in the control of cell proliferation. Dev Biol. 1987 Dec;124(2):295–308. doi: 10.1016/0012-1606(87)90483-0. [DOI] [PubMed] [Google Scholar]
- Zurier R. B., Kozma M., Sinnett-Smith J., Rozengurt E. Vasoactive intestinal peptide synergistically stimulates DNA synthesis in mouse 3T3 cells: role of cAMP, Ca2+, and protein kinase C. Exp Cell Res. 1988 May;176(1):155–161. doi: 10.1016/0014-4827(88)90129-2. [DOI] [PubMed] [Google Scholar]




