Abstract
1. The human recombinant somatostatin (SRIF) receptors, sst1 and sst2, have been stably expressed in mouse fibroblast (Ltk-) cells. Two stable clones, LSSR 1/20 and LSSR 11/13, expressing sst1 and sst2 receptors, respectively, have been used to characterize these receptor types using radioligand binding assays as well as measurements of changes in extracellular acidification rates using microphysiometry. 2. [125I]-[Tyr11]-SRIF bound to sst1 and sst2 receptors expressed in Ltk- cells with high affinity, Kd values being 1.52 nM, and 0.23 nM respectively. 3. In Ltk- cells expressing sst1 receptors, SRIF, SRIF-28, [D-Trp8]-SRIF and CGP 23996 all displaced [125I]-[Tyr11]-SRIF binding with high potency (IC50 values of 0.43 - 1.27 nM) whilst seglitide, BIM-23027, BIM-23056 and L-362855 were either weak inhibitors of binding or were ineffective. 4. In contrast MK-678 (seglitide) and BIM-23027 were the most potent inhibitors of [125I]-[Tyr11]-SRIF binding in Ltk- cells expressing sst2 receptors with IC50 values of 0.014 and 0.035 nM, respectively. 5. SRIF and a number of SRIF agonists, including seglitide and BIM-23027, caused concentration-dependent increases in extracellular acidification rates in Ltk- cells expressing sst2 receptors but not in Ltk- cells expressing sst1 receptors. The maximum increase in acidification rate produced by SRIF was 11.3 +/- 0.7% above baseline (0.1-0.28 pH unit min-1). The relative potencies of the SRIF agonists examined in causing increases in extracellular acidification rates in Ltk- cells expressing sst2 receptors correlated well with their relative potencies in inhibiting [125I]-[Tyr11] -SRIF binding (r = 0.94). 6. The increase in extracellular acidification produced by SRIF was markedly inhibited by pretreatment of cells with pertussis toxin (100 ng ml-1) indicating the involvement of pertussis toxin-sensitive G proteins. 7. SRIF (1 microM) had no effect on basal cyclic AMP levels in Ltk- cells expressing sst1 or sst2 receptors nor did it inhibit forskolin stimulated increases in cyclic AMP levels in either cell type. 8. The results from the present study describe the operational characteristics of human sst2 receptors expressed in Ltk- cells where receptor activation causes increases in extracellular acidification rates. This receptor is coupled to a pertussis toxin-sensitive G protein. In contrast, activation of sst1 receptors, at a similar transfection density, did not cause increases in extracellular acidification rates.
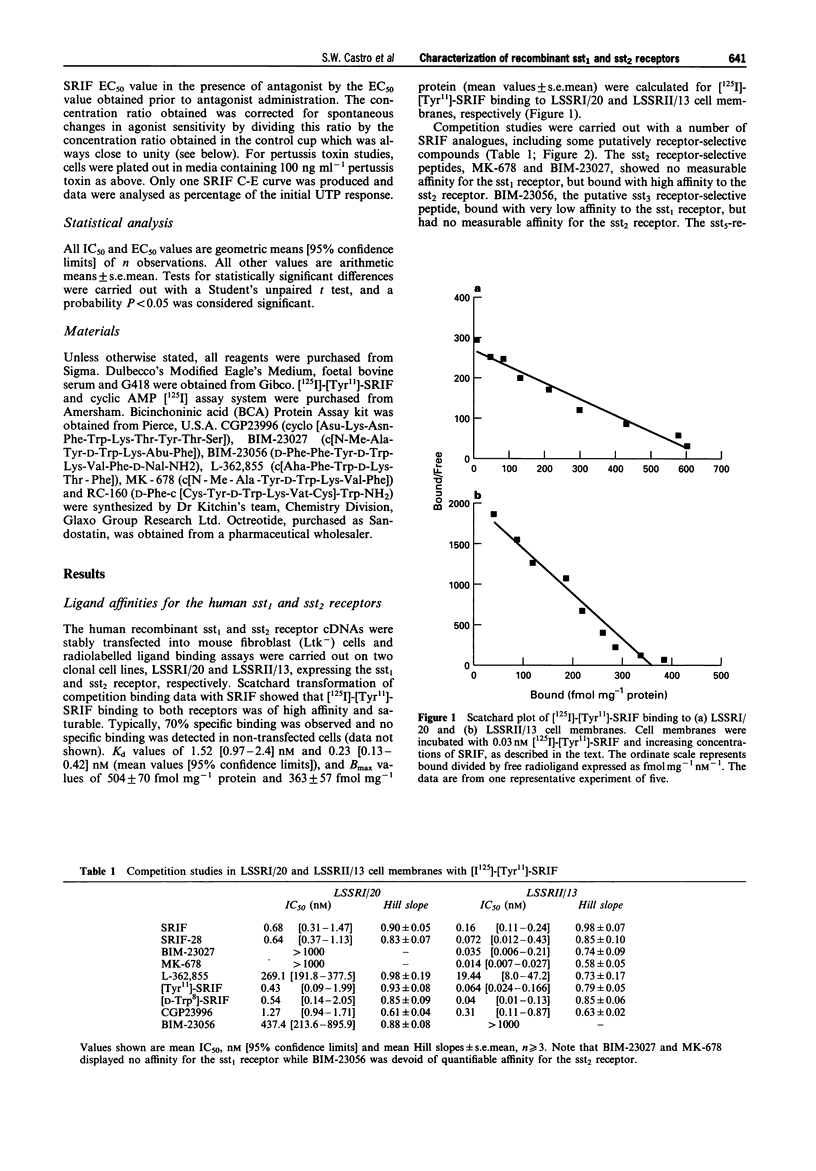
Full text
PDF

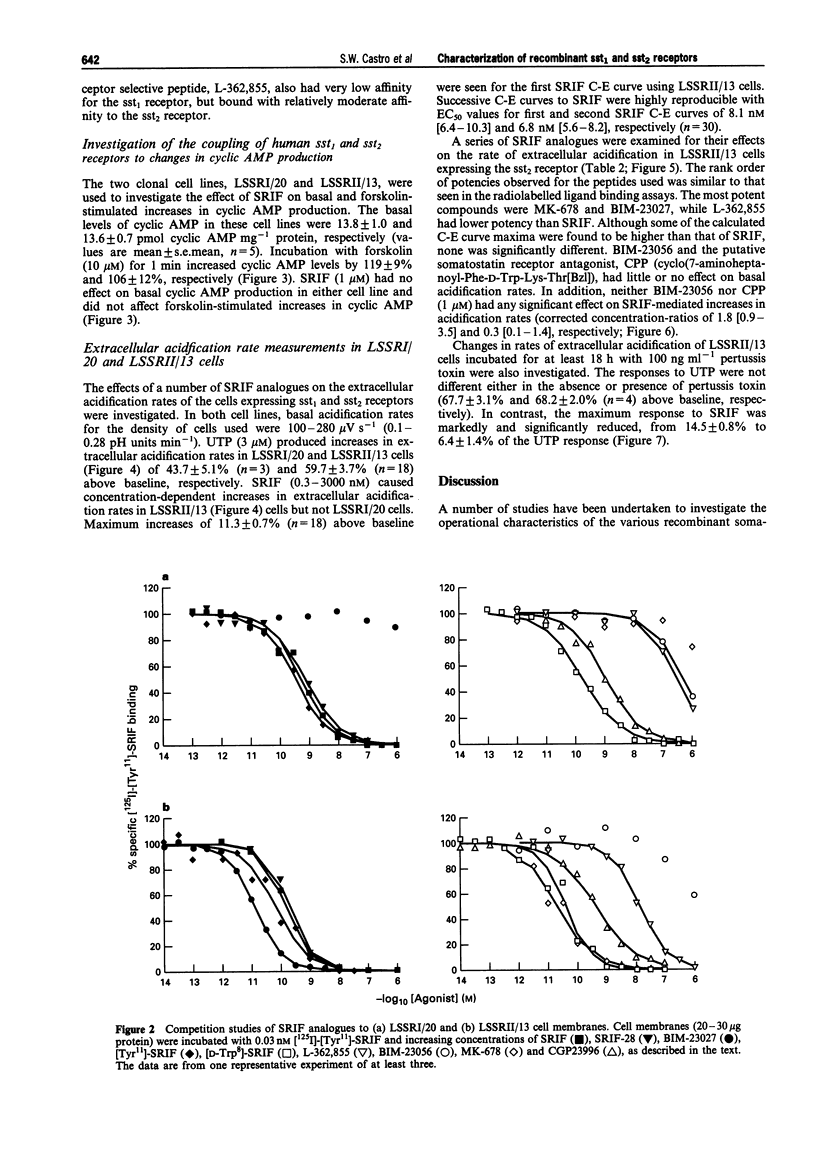


Selected References
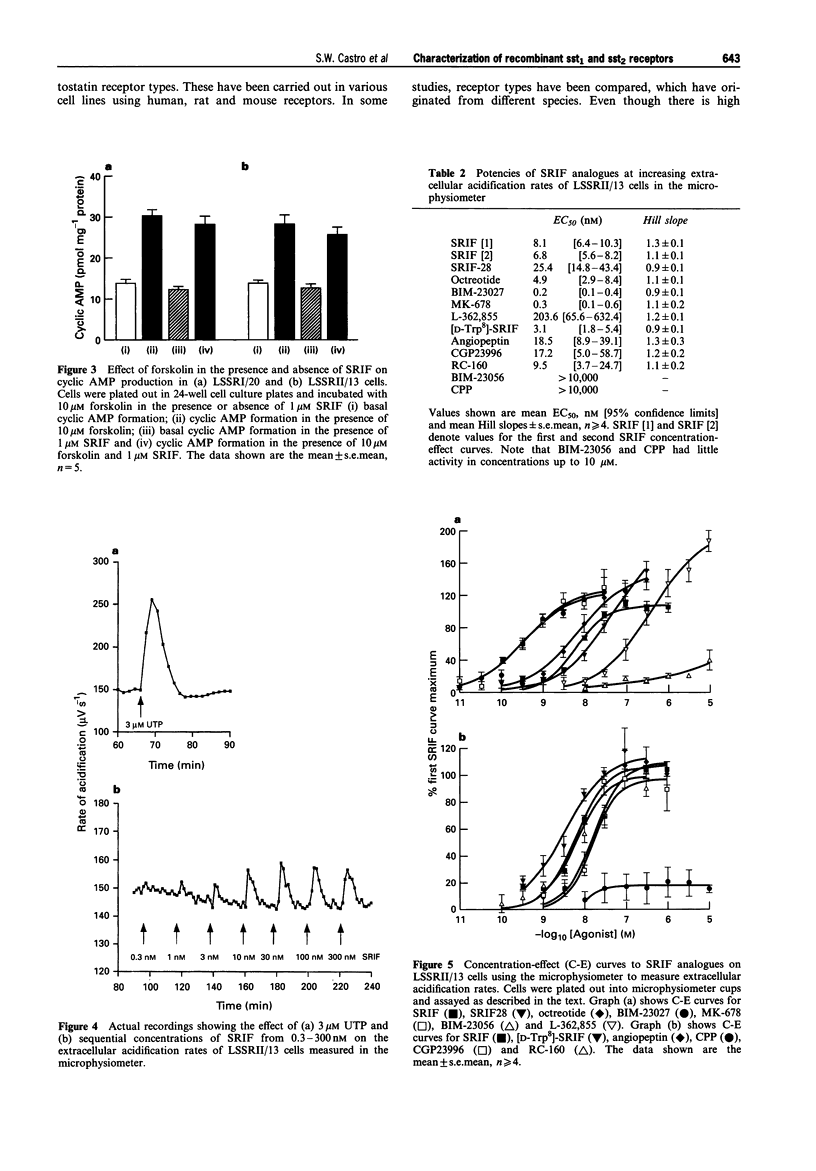
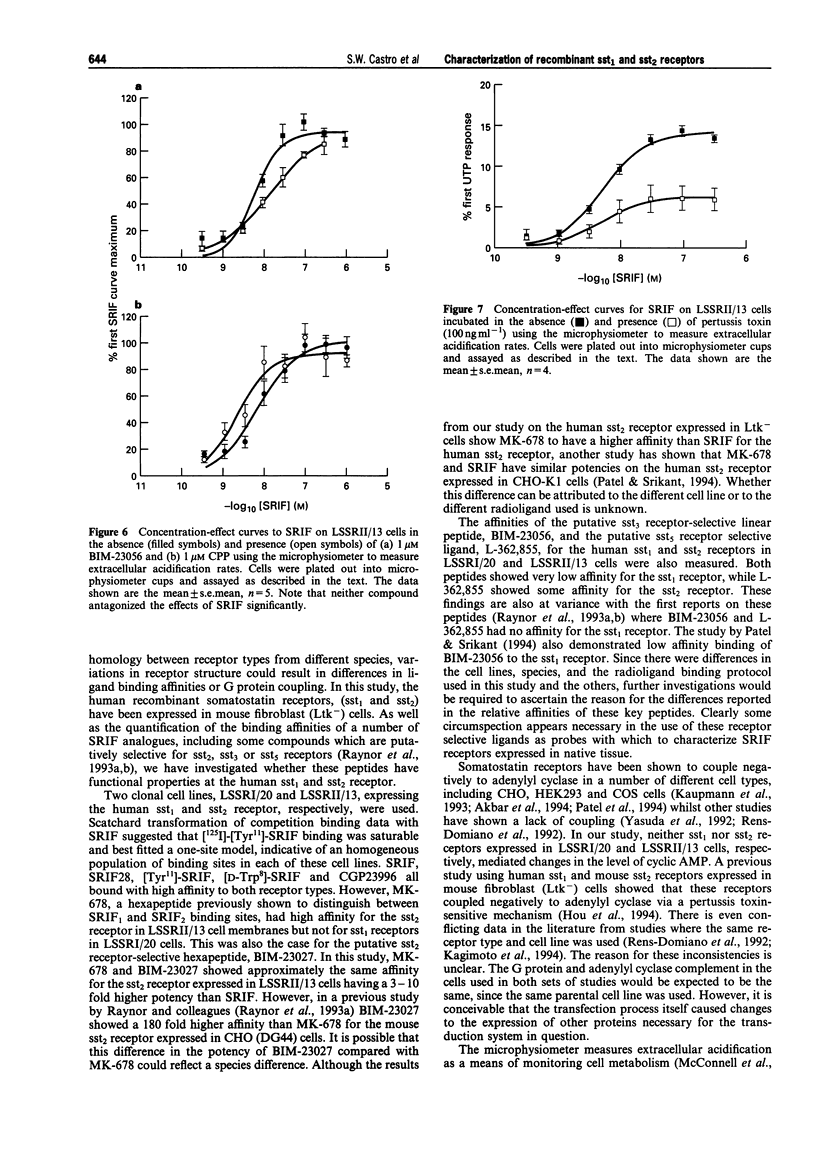
These references are in PubMed. This may not be the complete list of references from this article.
- Akbar M., Okajima F., Tomura H., Majid M. A., Yamada Y., Seino S., Kondo Y. Phospholipase C activation and Ca2+ mobilization by cloned human somatostatin receptor subtypes 1-5, in transfected COS-7 cells. FEBS Lett. 1994 Jul 11;348(2):192–196. doi: 10.1016/0014-5793(94)00603-2. [DOI] [PubMed] [Google Scholar]
- Barber D. L., McGuire M. E., Ganz M. B. Beta-adrenergic and somatostatin receptors regulate Na-H exchange independent of cAMP. J Biol Chem. 1989 Dec 15;264(35):21038–21042. [PubMed] [Google Scholar]
- Buell G., Schulz M. F., Arkinstall S. J., Maury K., Missotten M., Adami N., Talabot F., Kawashima E. Molecular characterisation, expression and localisation of human neurokinin-3 receptor. FEBS Lett. 1992 Mar 24;299(1):90–95. doi: 10.1016/0014-5793(92)80107-r. [DOI] [PubMed] [Google Scholar]
- Epelbaum J. Somatostatin in the central nervous system: physiology and pathological modifications. Prog Neurobiol. 1986;27(1):63–100. doi: 10.1016/0301-0082(86)90012-2. [DOI] [PubMed] [Google Scholar]
- Feniuk W., Dimech J., Jarvie E. M., Humphrey P. P. Further evidence from functional studies for somatostatin receptor heterogeneity in guinea-pig isolated ileum, vas deferens and right atrium. Br J Pharmacol. 1995 Jul;115(6):975–980. doi: 10.1111/j.1476-5381.1995.tb15906.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III creates targeted breakpoints for DNA sequencing. Gene. 1984 Jun;28(3):351–359. doi: 10.1016/0378-1119(84)90153-7. [DOI] [PubMed] [Google Scholar]
- Hou C., Gilbert R. L., Barber D. L. Subtype-specific signaling mechanisms of somatostatin receptors SSTR1 and SSTR2. J Biol Chem. 1994 Apr 8;269(14):10357–10362. [PubMed] [Google Scholar]
- Hoyer D., Bell G. I., Berelowitz M., Epelbaum J., Feniuk W., Humphrey P. P., O'Carroll A. M., Patel Y. C., Schonbrunn A., Taylor J. E. Classification and nomenclature of somatostatin receptors. Trends Pharmacol Sci. 1995 Mar;16(3):86–88. doi: 10.1016/s0165-6147(00)88988-9. [DOI] [PubMed] [Google Scholar]
- Jakobs K. H., Aktories K., Schultz G. A nucleotide regulatory site for somatostatin inhibition of adenylate cyclase in S49 lymphoma cells. Nature. 1983 May 12;303(5913):177–178. doi: 10.1038/303177a0. [DOI] [PubMed] [Google Scholar]
- Kagimoto S., Yamada Y., Kubota A., Someya Y., Ihara Y., Yasuda K., Kozasa T., Imura H., Seino S., Seino Y. Human somatostatin receptor, SSTR2, is coupled to adenylyl cyclase in the presence of Gi alpha 1 protein. Biochem Biophys Res Commun. 1994 Jul 29;202(2):1188–1195. doi: 10.1006/bbrc.1994.2054. [DOI] [PubMed] [Google Scholar]
- Kaupmann K., Bruns C., Hoyer D., Seuwen K., Lübbert H. Distribution and second messenger coupling of four somatostatin receptor subtypes expressed in brain. FEBS Lett. 1993 Sep 27;331(1-2):53–59. doi: 10.1016/0014-5793(93)80296-7. [DOI] [PubMed] [Google Scholar]
- Koch B. D., Dorflinger L. J., Schonbrunn A. Pertussis toxin blocks both cyclic AMP-mediated and cyclic AMP-independent actions of somatostatin. Evidence for coupling of Ni to decreases in intracellular free calcium. J Biol Chem. 1985 Oct 25;260(24):13138–13145. [PubMed] [Google Scholar]
- Lewis D. L., Weight F. F., Luini A. A guanine nucleotide-binding protein mediates the inhibition of voltage-dependent calcium current by somatostatin in a pituitary cell line. Proc Natl Acad Sci U S A. 1986 Dec;83(23):9035–9039. doi: 10.1073/pnas.83.23.9035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebow C., Reilly C., Serrano M., Schally A. V. Somatostatin analogues inhibit growth of pancreatic cancer by stimulating tyrosine phosphatase. Proc Natl Acad Sci U S A. 1989 Mar;86(6):2003–2007. doi: 10.1073/pnas.86.6.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell H. M., Owicki J. C., Parce J. W., Miller D. L., Baxter G. T., Wada H. G., Pitchford S. The cytosensor microphysiometer: biological applications of silicon technology. Science. 1992 Sep 25;257(5078):1906–1912. doi: 10.1126/science.1329199. [DOI] [PubMed] [Google Scholar]
- McKeen E. S., Feniuk W., Humphrey P. P. Mediation by SRIF1 receptors of the contractile action of somatostatin in rat isolated distal colon; studies using some novel SRIF analogues. Br J Pharmacol. 1994 Oct;113(2):628–634. doi: 10.1111/j.1476-5381.1994.tb17036.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPherson G. A. Analysis of radioligand binding experiments. A collection of computer programs for the IBM PC. J Pharmacol Methods. 1985 Nov;14(3):213–228. doi: 10.1016/0160-5402(85)90034-8. [DOI] [PubMed] [Google Scholar]
- Munson P. J., Rodbard D. Ligand: a versatile computerized approach for characterization of ligand-binding systems. Anal Biochem. 1980 Sep 1;107(1):220–239. doi: 10.1016/0003-2697(80)90515-1. [DOI] [PubMed] [Google Scholar]
- Patel Y. C., Greenwood M. T., Warszynska A., Panetta R., Srikant C. B. All five cloned human somatostatin receptors (hSSTR1-5) are functionally coupled to adenylyl cyclase. Biochem Biophys Res Commun. 1994 Jan 28;198(2):605–612. doi: 10.1006/bbrc.1994.1088. [DOI] [PubMed] [Google Scholar]
- Patel Y. C., Srikant C. B. Subtype selectivity of peptide analogs for all five cloned human somatostatin receptors (hsstr 1-5). Endocrinology. 1994 Dec;135(6):2814–2817. doi: 10.1210/endo.135.6.7988476. [DOI] [PubMed] [Google Scholar]
- Raynor K., Murphy W. A., Coy D. H., Taylor J. E., Moreau J. P., Yasuda K., Bell G. I., Reisine T. Cloned somatostatin receptors: identification of subtype-selective peptides and demonstration of high affinity binding of linear peptides. Mol Pharmacol. 1993 Jun;43(6):838–844. [PubMed] [Google Scholar]
- Raynor K., O'Carroll A. M., Kong H., Yasuda K., Mahan L. C., Bell G. I., Reisine T. Characterization of cloned somatostatin receptors SSTR4 and SSTR5. Mol Pharmacol. 1993 Aug;44(2):385–392. [PubMed] [Google Scholar]
- Raynor K., Reisine T. Analogs of somatostatin selectively label distinct subtypes of somatostatin receptors in rat brain. J Pharmacol Exp Ther. 1989 Nov;251(2):510–517. [PubMed] [Google Scholar]
- Raynor K., Wang H. L., Dichter M., Reisine T. Subtypes of brain somatostatin receptors couple to multiple cellular effector systems. Mol Pharmacol. 1991 Aug;40(2):248–253. [PubMed] [Google Scholar]
- Reichlin S. Somatostatin (second of two parts). N Engl J Med. 1983 Dec 22;309(25):1556–1563. doi: 10.1056/NEJM198312223092506. [DOI] [PubMed] [Google Scholar]
- Rens-Domiano S., Law S. F., Yamada Y., Seino S., Bell G. I., Reisine T. Pharmacological properties of two cloned somatostatin receptors. Mol Pharmacol. 1992 Jul;42(1):28–34. [PubMed] [Google Scholar]
- Reubi J. C. Evidence for two somatostatin-14 receptor types in rat brain cortex. Neurosci Lett. 1984 Aug 31;49(3):259–263. doi: 10.1016/0304-3940(84)90299-4. [DOI] [PubMed] [Google Scholar]
- Viguerie N., Tahiri-Jouti N., Ayral A. M., Cambillau C., Scemama J. L., Bastié M. J., Knuhtsen S., Estève J. P., Pradayrol L., Susini C. Direct inhibitory effects of a somatostatin analog, SMS 201-995, on AR4-2J cell proliferation via pertussis toxin-sensitive guanosine triphosphate-binding protein-independent mechanism. Endocrinology. 1989 Feb;124(2):1017–1025. doi: 10.1210/endo-124-2-1017. [DOI] [PubMed] [Google Scholar]
- Yamada Y., Post S. R., Wang K., Tager H. S., Bell G. I., Seino S. Cloning and functional characterization of a family of human and mouse somatostatin receptors expressed in brain, gastrointestinal tract, and kidney. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):251–255. doi: 10.1073/pnas.89.1.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita N., Shibuya N., Ogata E. Hyperpolarization of the membrane potential caused by somatostatin in dissociated human pituitary adenoma cells that secrete growth hormone. Proc Natl Acad Sci U S A. 1986 Aug;83(16):6198–6202. doi: 10.1073/pnas.83.16.6198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda K., Rens-Domiano S., Breder C. D., Law S. F., Saper C. B., Reisine T., Bell G. I. Cloning of a novel somatostatin receptor, SSTR3, coupled to adenylylcyclase. J Biol Chem. 1992 Oct 5;267(28):20422–20428. [PubMed] [Google Scholar]


