Abstract
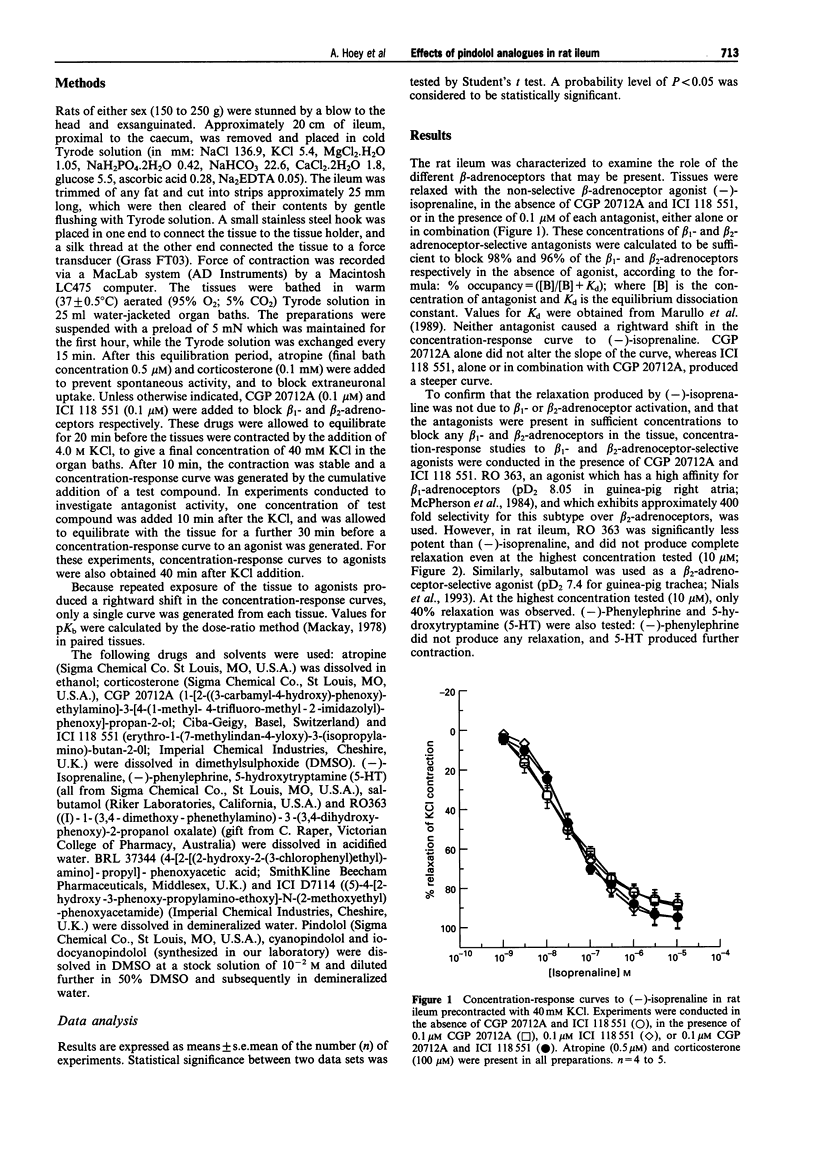
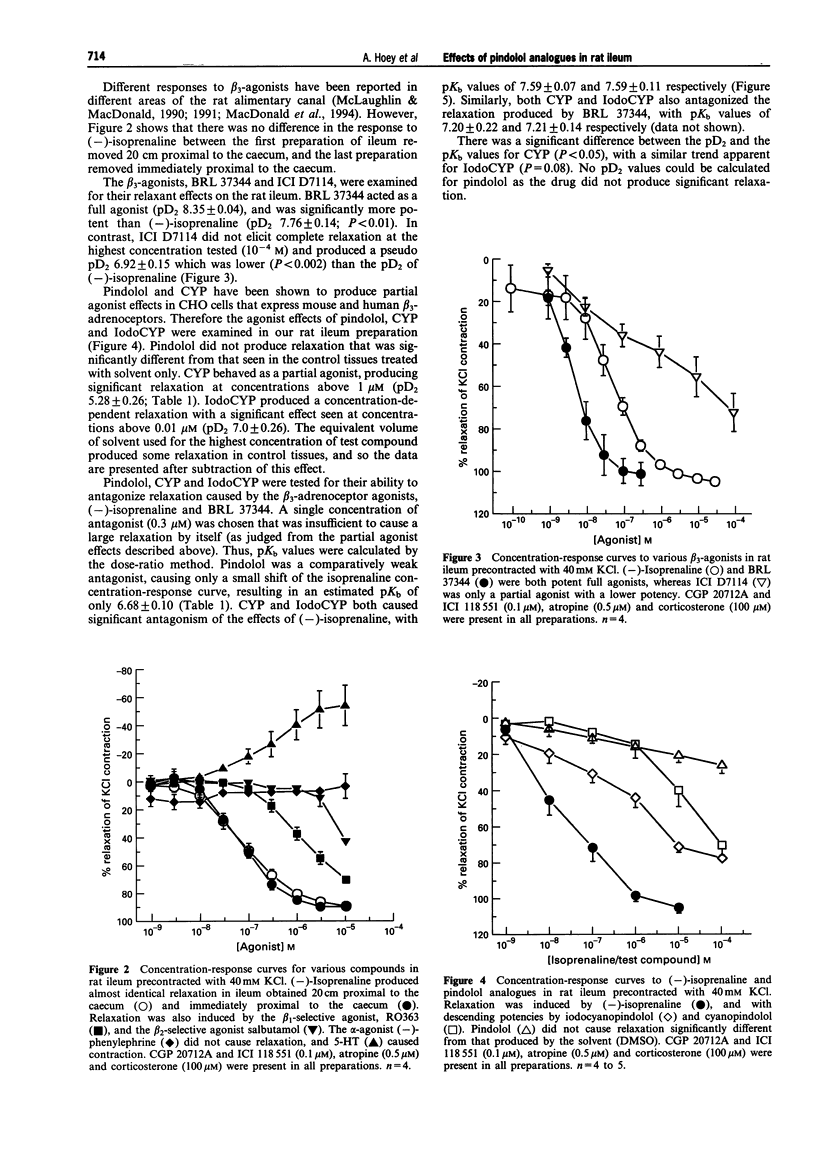
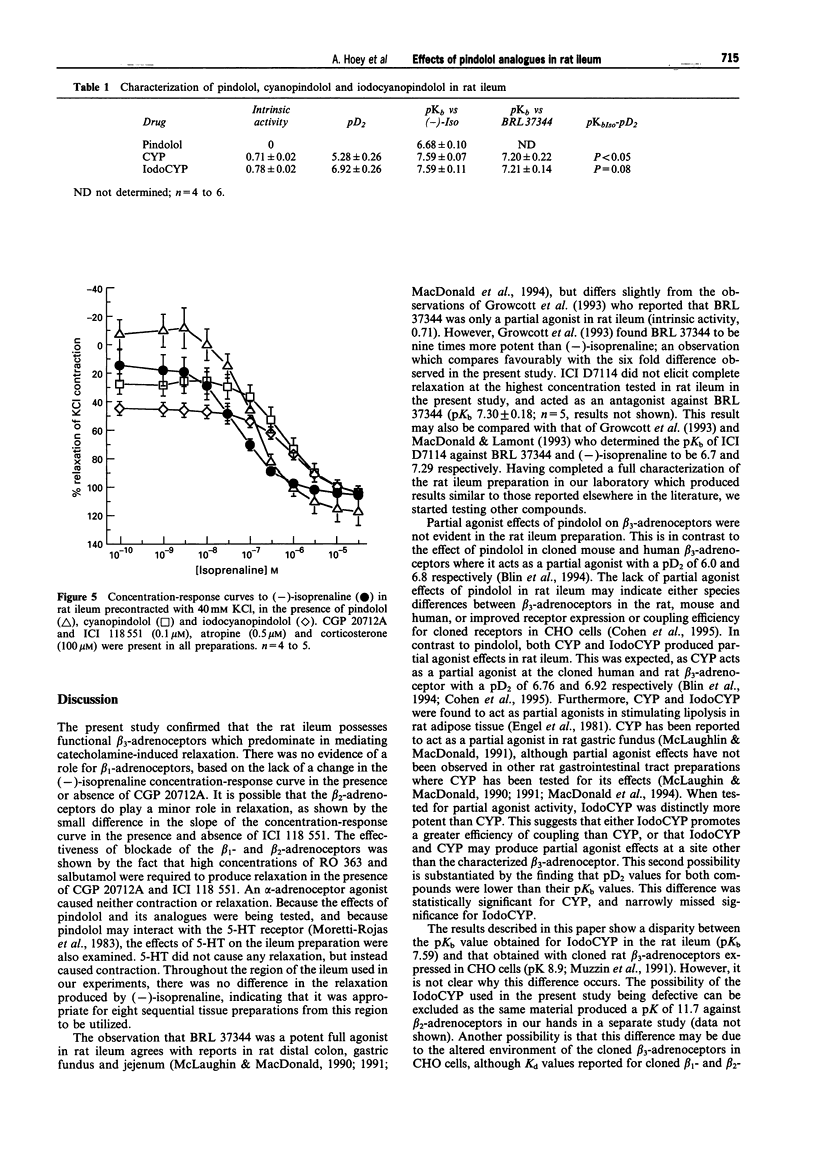
1. Pindolol, cyanopindolol (CYP) and iodocyanopindolol (IodoCYP) have been reported to act either as antagonists, agonists or partial agonists at the beta 3-adrenoceptor in different preparations. A comprehensive investigation has not yet been described with these compounds tested in one tissue from one species. This study was conducted to delineate the pharmacological effects of pindolol, CYP and IodoCYP and to provide data on their affinities at the predominant beta-adrenoceptor in rat ileum. 2. The beta-adrenoceptors present in rat ileum were characterized in the presence of CGP 20712A and ICI 118 551, atropine and corticosterone, with (-)-isoprenaline used as an agonist. The role of the beta 1 and beta 2-adrenoceptors was determined by the omission of either CGP 20712A, ICI 118 551, or both, from the buffers. Conversely, the effectiveness of the beta 1- and beta 2-adrenoceptor blockade was examined by use of the beta 1-adrenoceptor-selective agonist, RO 363 and the beta 2-adrenoceptor-selective agonist, salbutamol. 3. There was no evidence for the presence of functional beta 1-adrenoceptors, and no strong evidence that beta 2-adrenoceptor stimulation contributed to the relaxant effects of (-)-isoprenaline. (-)-Phenylephrine did not produce relaxation of the tissue and 5-hydroxytryptamine produced contraction. 4. The beta 3-adrenoceptor-selective agonist, BRL 37344 and (-)-isoprenaline were potent full agonists (pD2 8.35 +/- 0.04 and 7.76 +/- 0.14 respectively), whereas ICI D7114 was less potent (pseudo pD2 6.92 +/- 0.15). These results indicate that the predominant functional beta-adrenoceptors in rat ileum are beta 3-adrenoceptors. 5. Partial agonist effects were produced by CYP (pD2 5.28 +/- 0.26) and IodoCYP (pD2 7.0 +/- 0.26), but not pindolol. All three compounds antagonized the effects of (-)-isoprenaline with pKb values of 6.68 +/- 0.10, 7.59 +/- 0.07 and 7.59 +/- 0.11 for pindolol, CYP and IodoCYP respectively. Likewise, CYP and IodoCYP antagonized the effects of BRL 37344 with pKb values of 7.20 +/- 0.22 and 7.21 +/- 0.14 respectively. This study provides the first functional data on the effects of IodoCYP, the ligand with the highest known affinity for the beta 3-adrenoceptor, at the characterized rat ileum beta 3-adrenoceptor. 6. In conclusion, whereas pKb values suggest that CYP and IodoCYP have a similar affinity for the beta 3-adrenoceptor in rat ileum, the higher potency of IodoCYP suggests that it promotes a greater coupling efficiency, or that its partial agonist effects are produced through a site other than the beta 3-adrenoceptor. The similar pKb values for CYP and IodoCYP at the beta 3-adrenoceptor contrast with their order of known affinities at the beta 1- and beta 2-adrenoceptors, where IodoCYP is far more potent than CYP. This provides evidence of further differences in the characteristics of the beta 3-adrenoceptors compared to the beta 1- and beta 2-adrenoceptors. Finally, the utility of IodoCYP as a beta 3-adrenoceptor antagonist would appear to be limited because of the greater magnitude of partial agonist effects that it produces.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blin N., Camoin L., Maigret B., Strosberg A. D. Structural and conformational features determining selective signal transduction in the beta 3-adrenergic receptor. Mol Pharmacol. 1993 Dec;44(6):1094–1104. [PubMed] [Google Scholar]
- Brown L., Deighton N. M., Bals S., Söhlmann W., Zerkowski H. R., Michel M. C., Brodde O. E. Spare receptors for beta-adrenoceptor-mediated positive inotropic effects of catecholamines in the human heart. J Cardiovasc Pharmacol. 1992 Feb;19(2):222–232. doi: 10.1097/00005344-199202000-00011. [DOI] [PubMed] [Google Scholar]
- Carpéné C., Ambid L., Lafontan M. Predominance of beta 3-adrenergic component in catecholamine activation of lipolysis in garden dormouse adipocytes. Am J Physiol. 1994 Mar;266(3 Pt 2):R896–R904. doi: 10.1152/ajpregu.1994.266.3.R896. [DOI] [PubMed] [Google Scholar]
- Cohen M. L., Granneman J. G., Chaudhry A., Schenck K. W., Cushing D. J., Palkowitz A. D. Is the "atypical" beta-receptor in the rat stomach fundus the rat beta 3 receptor? J Pharmacol Exp Ther. 1995 Jan;272(1):446–451. [PubMed] [Google Scholar]
- Cook N. S., Weir S. W., Danzeisen M. C. Anti-vasoconstrictor effects of the K+ channel opener cromakalim on the rabbit aorta--comparison with the calcium antagonist isradipine. Br J Pharmacol. 1988 Nov;95(3):741–752. doi: 10.1111/j.1476-5381.1988.tb11700.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel G., Hoyer D., Berthold R., Wagner H. (+/-)[125Iodo] cyanopindolol, a new ligand for beta-adrenoceptors: identification and quantitation of subclasses of beta-adrenoceptors in guinea pig. Naunyn Schmiedebergs Arch Pharmacol. 1981;317(4):277–285. doi: 10.1007/BF00501307. [DOI] [PubMed] [Google Scholar]
- Growcott J. W., Holloway B., Green M., Wilson C. Zeneca ZD7114 acts as an antagonist at beta 3-adrenoceptors in rat isolated ileum. Br J Pharmacol. 1993 Dec;110(4):1375–1380. doi: 10.1111/j.1476-5381.1993.tb13972.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald A., Forbes I. J., Gallacher D., Heeps G., McLaughlin D. P. Adrenoceptors mediating relaxation to catecholamines in rat isolated jejunum. Br J Pharmacol. 1994 Jun;112(2):576–578. doi: 10.1111/j.1476-5381.1994.tb13113.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald A., Lamont M. Effects of selective antagonism of beta-adrenoceptor sub-types on responses to isoprenaline in rat distal colon in vitro. Br J Pharmacol. 1993 Dec;110(4):1551–1555. doi: 10.1111/j.1476-5381.1993.tb14000.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKay D. How should values of pA2 and affinity constants for pharmacological competitive antagonists be estimated? J Pharm Pharmacol. 1978 May;30(5):312–313. doi: 10.1111/j.2042-7158.1978.tb13237.x. [DOI] [PubMed] [Google Scholar]
- McLaughlin D. P., MacDonald A. Characterization of catecholamine-mediated relaxations in rat isolated gastric fundus: evidence for an atypical beta-adrenoceptor. Br J Pharmacol. 1991 Jun;103(2):1351–1356. doi: 10.1111/j.1476-5381.1991.tb09792.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin D. P., MacDonald A. Evidence for the existence of 'atypical' beta-adrenoceptors (beta 3-adrenoceptors) mediating relaxation in the rat distal colon in vitro. Br J Pharmacol. 1990 Nov;101(3):569–574. doi: 10.1111/j.1476-5381.1990.tb14122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPherson G. A., Malta E., Molenaar P., Raper C. The affinity and efficacy of the selective beta 1-adrenoceptor stimulant RO363 at beta 1- and beta 2-adrenoceptor sites. Br J Pharmacol. 1984 Aug;82(4):897–904. doi: 10.1111/j.1476-5381.1984.tb16488.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meunier H., Labrie F. Specificity of the beta 2-adrenergic receptor stimulating cyclic AMP accumulation in the intermediate lobe of rat pituitary gland. Eur J Pharmacol. 1982 Jul 16;81(3):411–420. doi: 10.1016/0014-2999(82)90106-6. [DOI] [PubMed] [Google Scholar]
- Moretti-Rojas I., Ezrailson E. G., Birnbaumer L., Entman M. L., Garber A. J. Serotonergic and adrenergic regulation of skeletal muscle metabolism in the rat. II. The use of [125I]iodolysergic acid diethylamide and [125I]iodopindolol as probes of sarcolemmal receptor function and specificity. J Biol Chem. 1983 Oct 25;258(20):12499–12508. [PubMed] [Google Scholar]
- Muzzin P., Revelli J. P., Kuhne F., Gocayne J. D., McCombie W. R., Venter J. C., Giacobino J. P., Fraser C. M. An adipose tissue-specific beta-adrenergic receptor. Molecular cloning and down-regulation in obesity. J Biol Chem. 1991 Dec 15;266(35):24053–24058. [PubMed] [Google Scholar]
- Neve K. A., McGonigle P., Molinoff P. B. Quantitative analysis of the selectivity of radioligands for subtypes of beta adrenergic receptors. J Pharmacol Exp Ther. 1986 Jul;238(1):46–53. [PubMed] [Google Scholar]
- Nials A. T., Sumner M. J., Johnson M., Coleman R. A. Investigations into factors determining the duration of action of the beta 2-adrenoceptor agonist, salmeterol. Br J Pharmacol. 1993 Feb;108(2):507–515. doi: 10.1111/j.1476-5381.1993.tb12833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


