Abstract
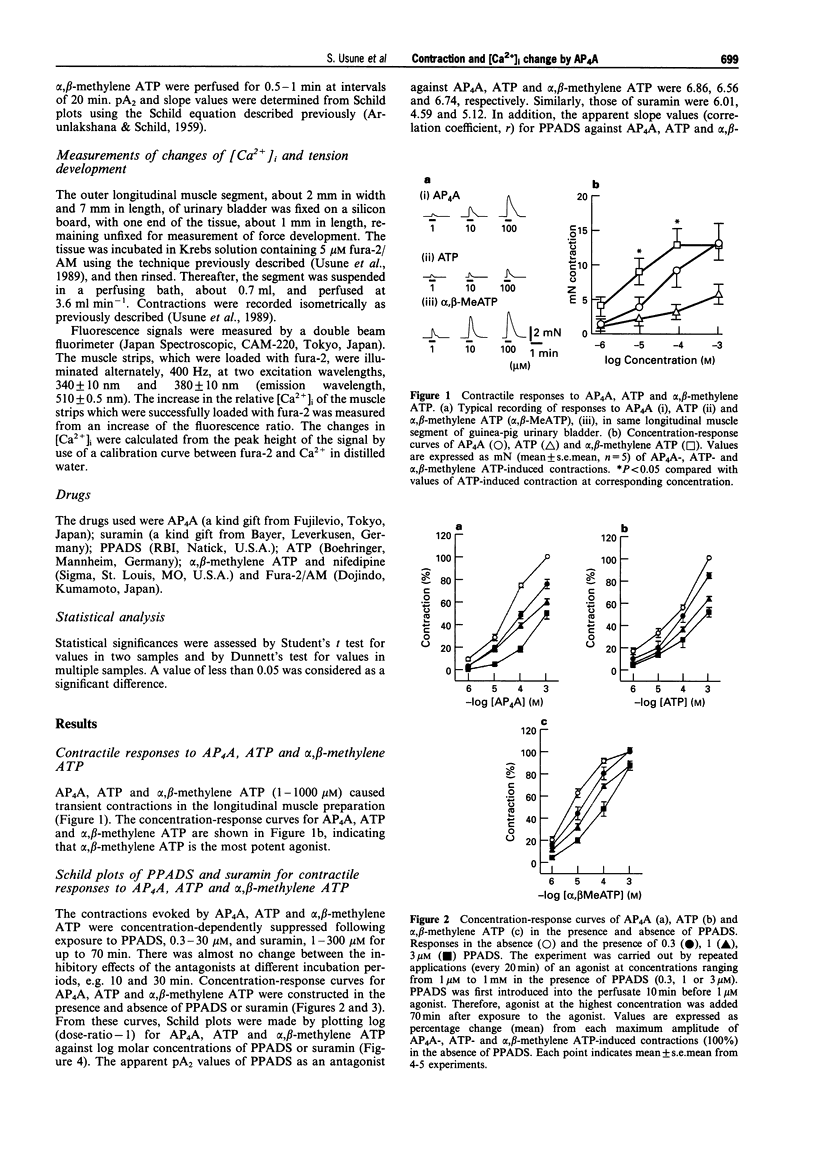
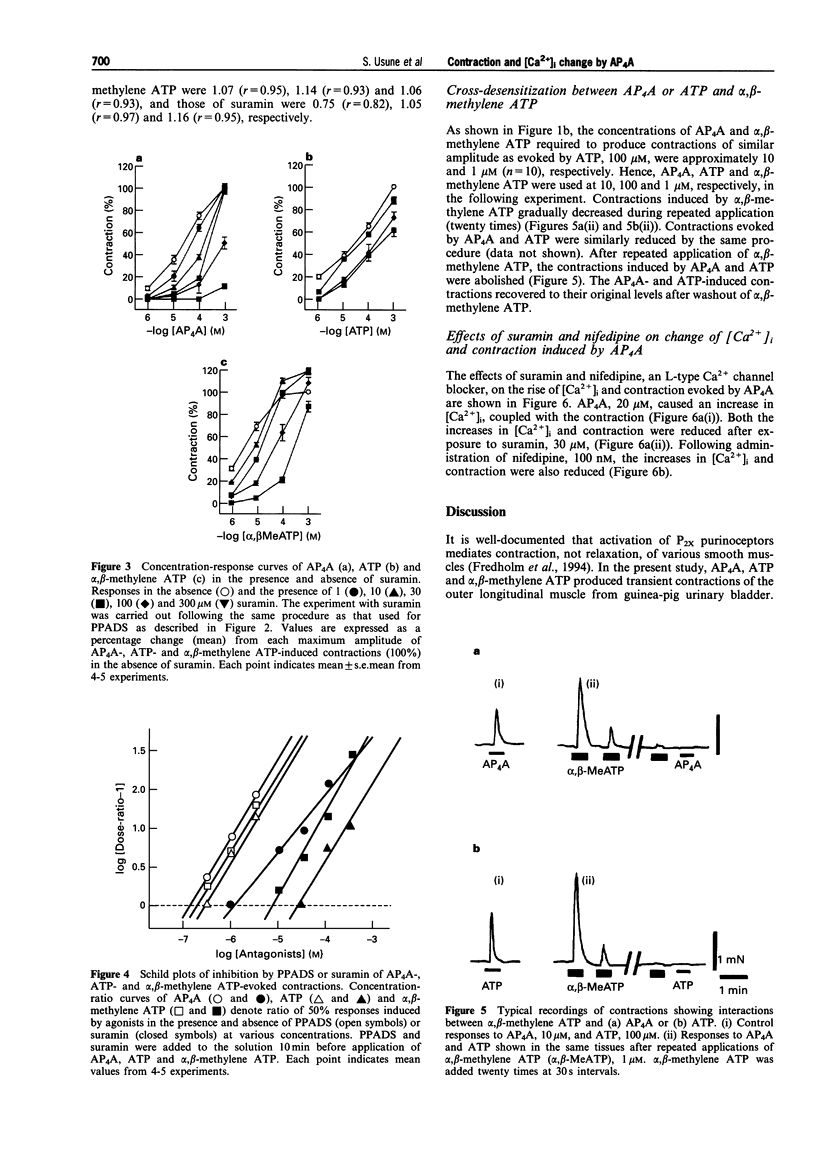
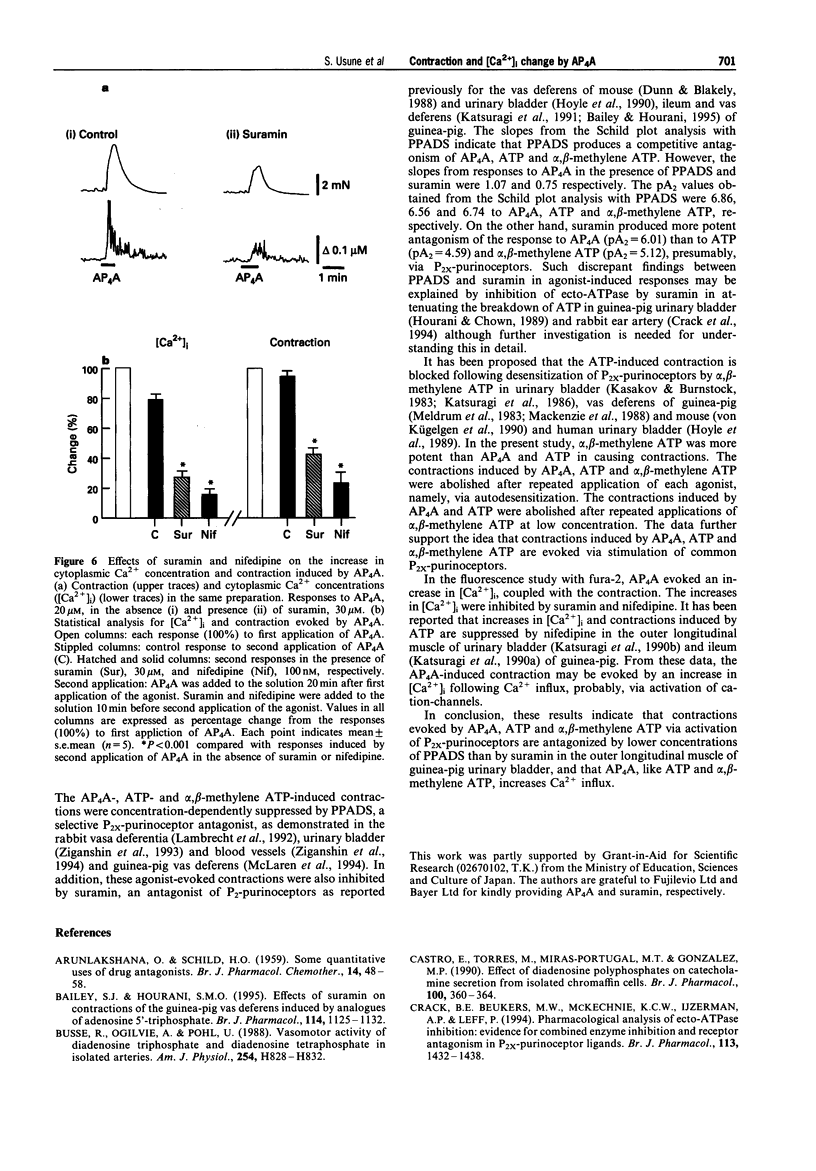
1. The contraction and intracellular Ca2+ change evoked by diadenosine tetraphosphate (AP4A) were studied in the outer longitudinal muscle of the guinea-pig urinary bladder and compared with those evoked by ATP and alpha, beta-methylene ATP (a P2-purinoceptor agonist). 2. AP4A, ATP and alpha, beta-methylene ATP produced concentration-dependent transient contractions. These contractions were inhibited by PPADS (pyridoralphosphate-6-azophenyl- 2'-4'-disulphonic acid), 0.3- 30 microM, a P2x-purinoceptor antagonist, and suramin, 1-300 microM, a P2-purinoceptor antagonist in a concentration-dependent manner. From Schild plot analysis, the apparent pA2 values for PPADS for contractions evoked by AP4A, ATP and alpha, beta-methylene ATP were 6.86, 6.56, 6.74, and those for suramin were 6.01, 4.59 and 5.12, respectively; the Schild slopes for PPADS were 1.07, 1.14 and 1.06, and, those for suramin 0.75, 1.05 and 1.16, respectively. 3. AP4A (10 microM) and ATP (100 microM) failed to elicit any contraction of the tissue after a desensitization produced by repeated application of alpha, beta-methylene ATP (1 microM). 4. In fluorescence experiments with fura-2, the increases in [Ca2+]i and contraction evoked by AP4A were suppressed by suramin and nifedipine, an L-type Ca2+ channel blocker. 5. These findings suggest that P2x-purinoceptors, which are more sensitive to PPADS than suramin, exist on the outer longitudinal muscles of guinea-pig urinary bladder, and that the AP4A-evoked contraction results from Ca2+ influx.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARUNLAKSHANA O., SCHILD H. O. Some quantitative uses of drug antagonists. Br J Pharmacol Chemother. 1959 Mar;14(1):48–58. doi: 10.1111/j.1476-5381.1959.tb00928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey S. J., Hourani S. M. Effects of suramin on contractions of the guinea-pig vas deferens induced by analogues of adenosine 5'-triphosphate. Br J Pharmacol. 1995 Mar;114(6):1125–1132. doi: 10.1111/j.1476-5381.1995.tb13324.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busse R., Ogilvie A., Pohl U. Vasomotor activity of diadenosine triphosphate and diadenosine tetraphosphate in isolated arteries. Am J Physiol. 1988 May;254(5 Pt 2):H828–H832. doi: 10.1152/ajpheart.1988.254.5.H828. [DOI] [PubMed] [Google Scholar]
- Castro E., Torres M., Miras-Portugal M. T., Gonzalez M. P. Effect of diadenosine polyphosphates on catecholamine secretion from isolated chromaffin cells. Br J Pharmacol. 1990 Jun;100(2):360–364. doi: 10.1111/j.1476-5381.1990.tb15809.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crack B. E., Beukers M. W., McKechnie K. C., Ijzerman A. P., Leff P. Pharmacological analysis of ecto-ATPase inhibition: evidence for combined enzyme inhibition and receptor antagonism in P2X-purinoceptor ligands. Br J Pharmacol. 1994 Dec;113(4):1432–1438. doi: 10.1111/j.1476-5381.1994.tb17157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn P. M., Blakeley A. G. Suramin: a reversible P2-purinoceptor antagonist in the mouse vas deferens. Br J Pharmacol. 1988 Feb;93(2):243–245. doi: 10.1111/j.1476-5381.1988.tb11427.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredholm B. B., Abbracchio M. P., Burnstock G., Daly J. W., Harden T. K., Jacobson K. A., Leff P., Williams M. Nomenclature and classification of purinoceptors. Pharmacol Rev. 1994 Jun;46(2):143–156. [PMC free article] [PubMed] [Google Scholar]
- Hourani S. M., Chown J. A. The effects of some possible inhibitors of ectonucleotidases on the breakdown and pharmacological effects of ATP in the guinea-pig urinary bladder. Gen Pharmacol. 1989;20(4):413–416. doi: 10.1016/0306-3623(89)90188-2. [DOI] [PubMed] [Google Scholar]
- Hoyle C. H., Chapple C., Burnstock G. Isolated human bladder: evidence for an adenine dinucleotide acting on P2X-purinoceptors and for purinergic transmission. Eur J Pharmacol. 1989 Dec 12;174(1):115–118. doi: 10.1016/0014-2999(89)90881-9. [DOI] [PubMed] [Google Scholar]
- Hoyle C. H., Knight G. E., Burnstock G. Suramin antagonizes responses to P2-purinoceptor agonists and purinergic nerve stimulation in the guinea-pig urinary bladder and taenia coli. Br J Pharmacol. 1990 Mar;99(3):617–621. doi: 10.1111/j.1476-5381.1990.tb12979.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasakov L., Burnstock G. The use of the slowly degradable analog, alpha, beta-methylene ATP, to produce desensitisation of the P2-purinoceptor: effect on non-adrenergic, non-cholinergic responses of the guinea-pig urinary bladder. Eur J Pharmacol. 1982 Dec 24;86(2):291–294. doi: 10.1016/0014-2999(82)90330-2. [DOI] [PubMed] [Google Scholar]
- Katsuragi T., Kuratomi L., Furukawa T. Clonidine-evoked selective P1-purinoceptor antagonism of contraction of guinea-pig urinary bladder. Eur J Pharmacol. 1986 Feb 11;121(1):119–122. doi: 10.1016/0014-2999(86)90400-0. [DOI] [PubMed] [Google Scholar]
- Katsuragi T., Tokunaga T., Ogawa S., Soejima O., Sato C., Furukawa T. Existence of ATP-evoked ATP release system in smooth muscles. J Pharmacol Exp Ther. 1991 Nov;259(2):513–518. [PubMed] [Google Scholar]
- Katsuragi T., Tokunaga T., Usune S., Furukawa T. A possible coupling of postjunctional ATP release and transmitters' receptor stimulation in smooth muscles. Life Sci. 1990;46(18):1301–1307. doi: 10.1016/0024-3205(90)90363-v. [DOI] [PubMed] [Google Scholar]
- Katsuragi T., Usune S., Furukawa T. Antagonism by nifedipine of contraction and Ca2(+)-influx evoked by ATP in guinea-pig urinary bladder. Br J Pharmacol. 1990 Jun;100(2):370–374. doi: 10.1111/j.1476-5381.1990.tb15811.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambrecht G., Friebe T., Grimm U., Windscheif U., Bungardt E., Hildebrandt C., Bäumert H. G., Spatz-Kümbel G., Mutschler E. PPADS, a novel functionally selective antagonist of P2 purinoceptor-mediated responses. Eur J Pharmacol. 1992 Jul 7;217(2-3):217–219. doi: 10.1016/0014-2999(92)90877-7. [DOI] [PubMed] [Google Scholar]
- MacKenzie I., Kirkpatrick K. A., Burnstock G. Comparative study of the actions of AP5A and alpha,beta-methylene ATP on nonadrenergic, noncholinergic neurogenic excitation in the guinea-pig vas deferens. Br J Pharmacol. 1988 Jul;94(3):699–706. doi: 10.1111/j.1476-5381.1988.tb11578.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaren G. J., Lambrecht G., Mutschler E., Bäumert H. G., Sneddon P., Kennedy C. Investigation of the actions of PPADS, a novel P2x-purinoceptor antagonist, in the guinea-pig isolated vas deferens. Br J Pharmacol. 1994 Mar;111(3):913–917. doi: 10.1111/j.1476-5381.1994.tb14825.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meldrum L. A., Burnstock G. Evidence that ATP acts as a co-transmitter with noradrenaline in sympathetic nerves supplying the guinea-pig vas deferens. Eur J Pharmacol. 1983 Aug 19;92(1-2):161–163. doi: 10.1016/0014-2999(83)90126-7. [DOI] [PubMed] [Google Scholar]
- Pintor J., Díaz-Rey M. A., Torres M., Miras-Portugal M. T. Presence of diadenosine polyphosphates--Ap4A and Ap5A--in rat brain synaptic terminals. Ca2+ dependent release evoked by 4-aminopyridine and veratridine. Neurosci Lett. 1992 Mar 2;136(2):141–144. doi: 10.1016/0304-3940(92)90034-5. [DOI] [PubMed] [Google Scholar]
- Pintor J., Rotllán P., Torres M., Miras-Portugal M. T. Characterization and quantification of diadenosine hexaphosphate in chromaffin cells: granular storage and secretagogue-induced release. Anal Biochem. 1992 Feb 1;200(2):296–300. doi: 10.1016/0003-2697(92)90469-n. [DOI] [PubMed] [Google Scholar]
- Pintor J., Torres M., Miras-Portugal M. T. Carbachol induced release of diadenosine polyphosphates--Ap4A and Ap5A--from perfused bovine adrenal medulla and isolated chromaffin cells. Life Sci. 1991;48(24):2317–2324. doi: 10.1016/0024-3205(91)90268-g. [DOI] [PubMed] [Google Scholar]
- Rodriguez del Castillo A., Torres M., Delicado E. G., Miras-Portugal M. T. Subcellular distribution studies of diadenosine polyphosphates--Ap4A and Ap5A--in bovine adrenal medulla: presence in chromaffin granules. J Neurochem. 1988 Dec;51(6):1696–1703. doi: 10.1111/j.1471-4159.1988.tb01147.x. [DOI] [PubMed] [Google Scholar]
- Usune S., Katsuragi T., Furukawa T. Two phases of the prostaglandin F2 alpha-induced contraction in guinea-pig taenia coli involve different Ca2+ channels. Naunyn Schmiedebergs Arch Pharmacol. 1989 Oct;340(4):437–441. doi: 10.1007/BF00167046. [DOI] [PubMed] [Google Scholar]
- Usune S., Katsuragi T., Sakamoto Y., Furukawa T. Contribution of Na+ and membrane depolarization to contraction induced by adrenaline in the guinea pig vas deferens. Can J Physiol Pharmacol. 1986 Jun;64(6):720–723. doi: 10.1139/y86-121. [DOI] [PubMed] [Google Scholar]
- Ziganshin A. U., Hoyle C. H., Bo X., Lambrecht G., Mutschler E., Bäumert H. G., Burnstock G. PPADS selectively antagonizes P2X-purinoceptor-mediated responses in the rabbit urinary bladder. Br J Pharmacol. 1993 Dec;110(4):1491–1495. doi: 10.1111/j.1476-5381.1993.tb13990.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziganshin A. U., Hoyle C. H., Lambrecht G., Mutschler E., Bümert H. G., Burnstock G. Selective antagonism by PPADS at P2X-purinoceptors in rabbit isolated blood vessels. Br J Pharmacol. 1994 Mar;111(3):923–929. doi: 10.1111/j.1476-5381.1994.tb14827.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Kügelgen I., Bültmann R., Starke K. Interaction of adenine nucleotides, UTP and suramin in mouse vas deferens: suramin-sensitive and suramin-insensitive components in the contractile effect of ATP. Naunyn Schmiedebergs Arch Pharmacol. 1990 Aug;342(2):198–205. doi: 10.1007/BF00166965. [DOI] [PubMed] [Google Scholar]


