Abstract
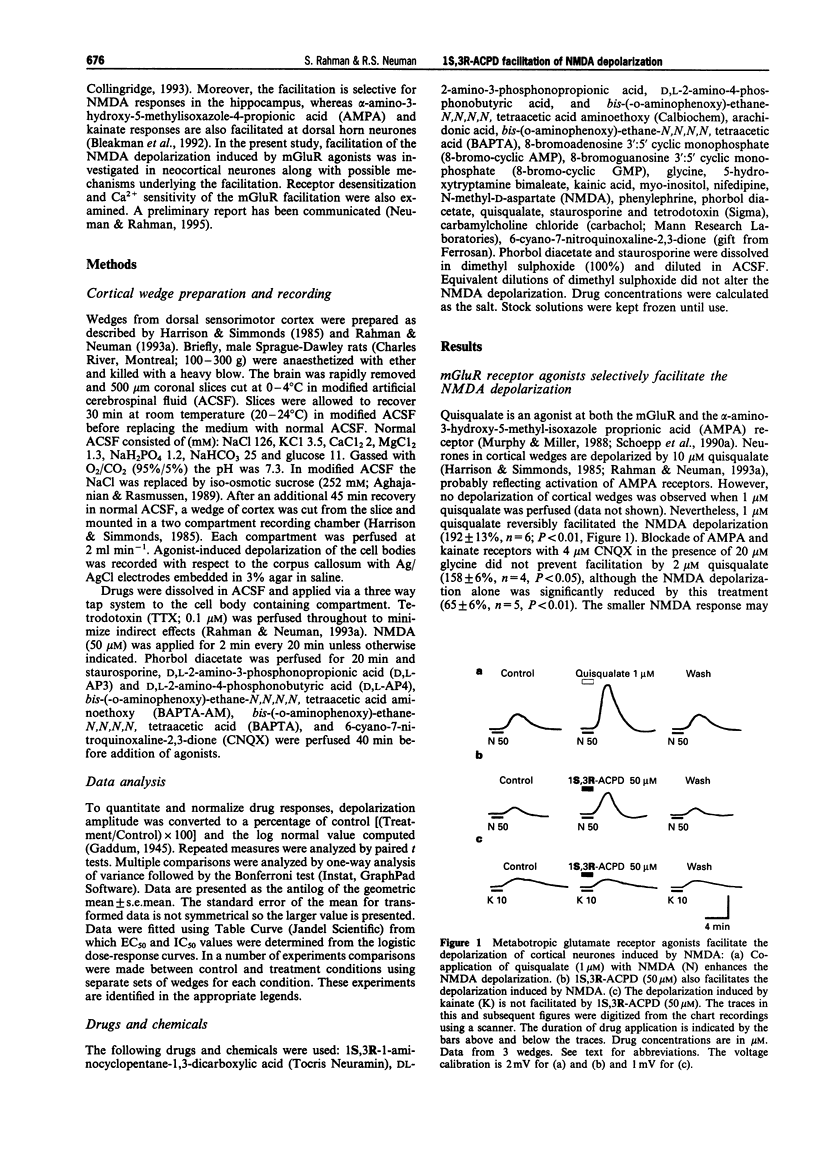
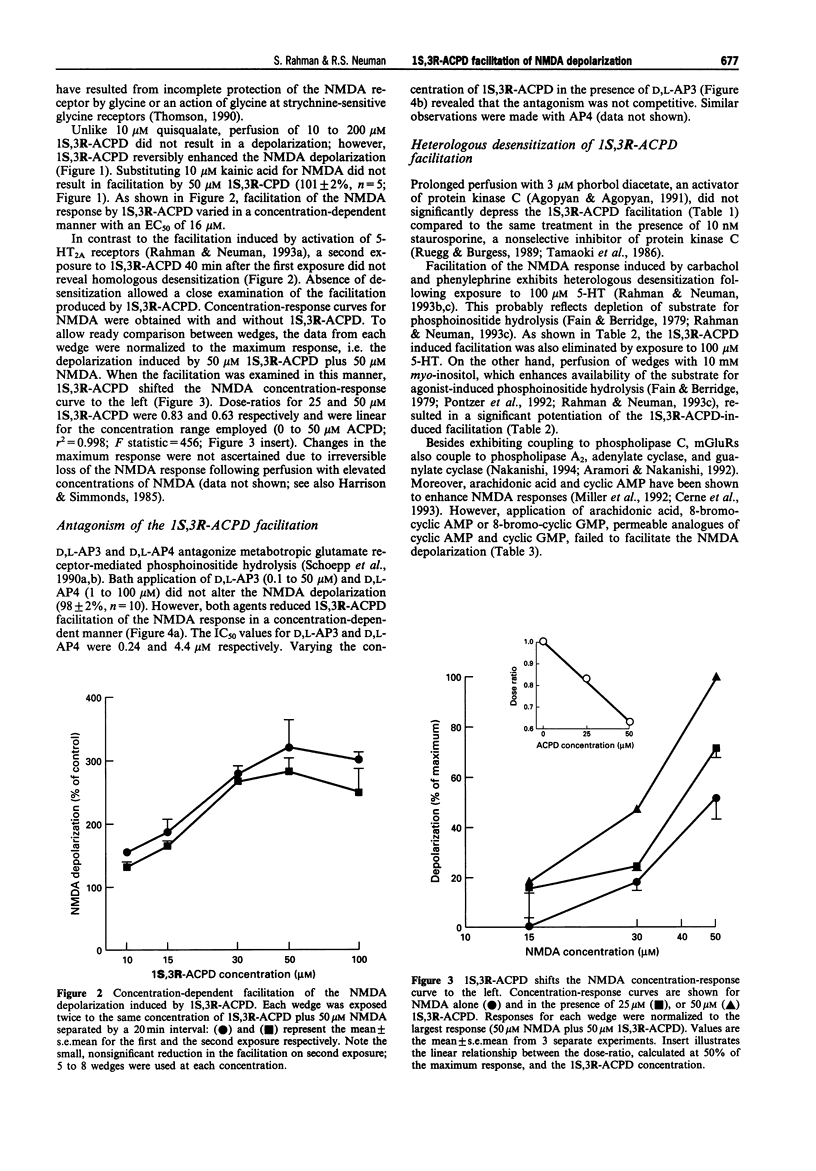
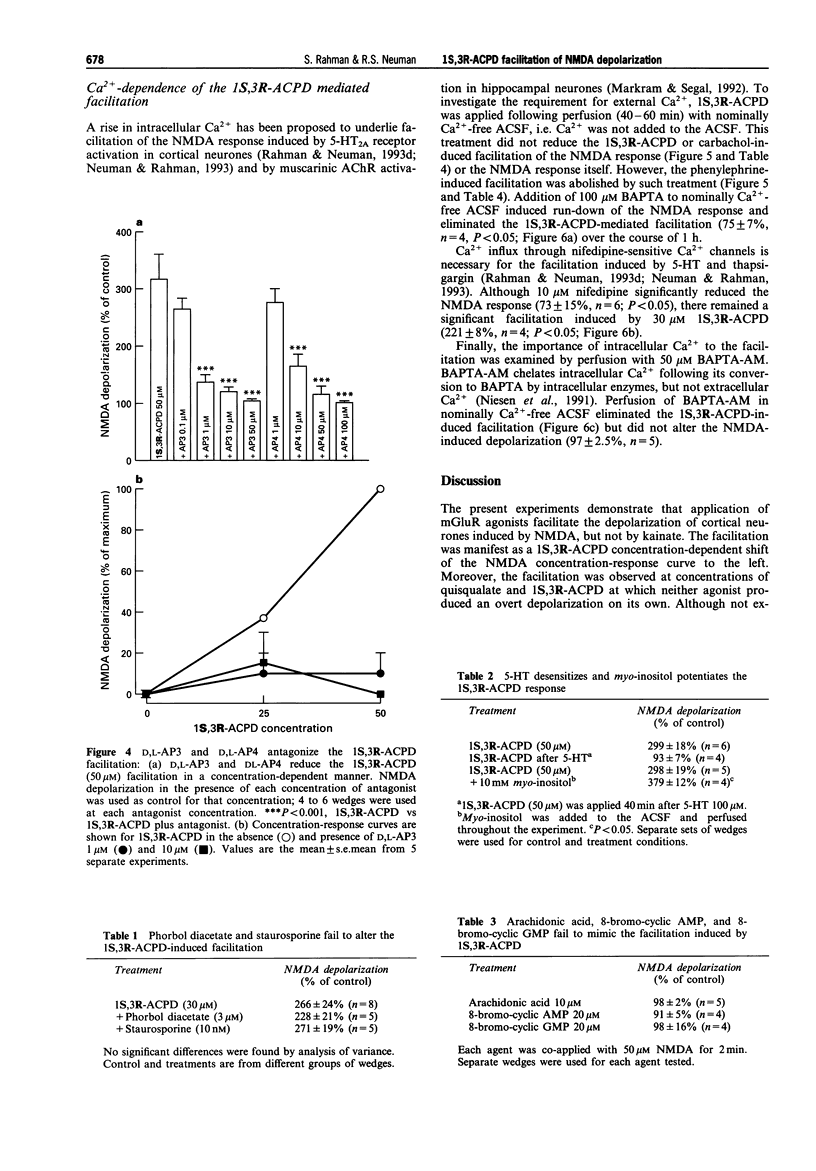
1. Facilitation of the N-methyl-D-aspartate (NMDA) receptor-mediated depolarization of cortical neurones induced by metabotropic glutamate receptor (mGluR) agonists in the presence of tetrodotoxin has been examined by use of grease-gap recording. 2. Quisqualate (1-2 microM) and 10 to 100 microM 1S,3R-I-aminocyclopentane-1,3-dicarboxylic acid (1S,3R-ACPD) facilitated the NMDA-, but not the kainate-induced depolarization with an EC50 of 16 microM for 1S,3R-ACPD. The facilitation induced by quisqualate was reduced, but not blocked, by 4 microM 6-cyano-7-nitroquinoxaline-2,3-dione. 3. D,L-2-Amino-3-phosphonopropionic acid and D,L-2-amino-4-phosphonobutyric acid antagonized the 1S,3R-ACPD facilitation in a non-competitive manner with IC50 values of 0.24 microM and 4.4 microM respectively. 4. Homologous desensitization of the 1S,3R-ACPD induced facilitation was not observed. The facilitation was not altered by 10 nM staurosporine or 3 microM phorbol diacetate. 5. Substitution of 20 microM 8-bromo-cyclic adenosine monophosphate, 20 microM 8-bromo-cyclic guanosine monophosphate, or 10 microM arachidonic acid for 1S,3R-ACPD did not induce facilitation of the NMDA response. However, the 1S,3R-ACPD facilitation was potentiated by 10 mM myo-inositol and exhibited heterologous desensitization following exposure to 100 microM 5-hydroxytryptamine. 6. The 1S,3R-ACPD-induced facilitation persisted in both 10 microM nifedipine and nominally Ca(2+)-free medium and was only gradually eliminated following addition of 100 microM bis-(-o-aminophenoxy)-ethane-N,N,N,N-tetraacetic acid in Ca(2+)-free medium. Facilitation of the NMDA response induced by carbachol, but not phenylephrine, was also observed in nominally Ca(2+)-free medium. Perfusing 50 microM bis-(-aminophenoxy)-ethane-N,N,N,N-tetraacetic acid aminoethoxy eliminated the 1S,3R-ACPD facilitation. 7. These experiments have shown that mGluR agonists selectively facilitate the NMDA depolarization of cortical wedges, most likely by activating one or more mGluR subtypes that couple to phospholipase C. We conclude the facilitation results from a Ca(2+)-sensitive mechanism dependent on activation of phospholipase C and release of internal Ca2+. The facilitation is not contingent on activation of protein kinase C or entry of Ca2+ through nifedipine-sensitive Ca2+ channels.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe T., Sugihara H., Nawa H., Shigemoto R., Mizuno N., Nakanishi S. Molecular characterization of a novel metabotropic glutamate receptor mGluR5 coupled to inositol phosphate/Ca2+ signal transduction. J Biol Chem. 1992 Jul 5;267(19):13361–13368. [PubMed] [Google Scholar]
- Aghajanian G. K., Rasmussen K. Intracellular studies in the facial nucleus illustrating a simple new method for obtaining viable motoneurons in adult rat brain slices. Synapse. 1989;3(4):331–338. doi: 10.1002/syn.890030406. [DOI] [PubMed] [Google Scholar]
- Agopyan N., Agopyan I. Effects of protein kinase C activators and inhibitors on membrane properties, synaptic responses, and cholinergic actions in CA1 subfield of rat hippocampus in situ and in vitro. Synapse. 1991 Mar;7(3):193–206. doi: 10.1002/syn.890070304. [DOI] [PubMed] [Google Scholar]
- Aniksztejn L., Bregestovski P., Ben-Ari Y. Selective activation of quisqualate metabotropic receptor potentiates NMDA but not AMPA responses. Eur J Pharmacol. 1991 Dec 3;205(3):327–328. doi: 10.1016/0014-2999(91)90921-c. [DOI] [PubMed] [Google Scholar]
- Aniksztejn Laurent, Otani Satoru, Ben-Ari Yehezkel. Quisqualate Metabotropic Receptors Modulate NMDA Currents and Facilitate Induction of Long-Term Potentiation Through Protein Kinase C. Eur J Neurosci. 1992;4(6):500–505. doi: 10.1111/j.1460-9568.1992.tb00900.x. [DOI] [PubMed] [Google Scholar]
- Aramori I., Nakanishi S. Signal transduction and pharmacological characteristics of a metabotropic glutamate receptor, mGluR1, in transfected CHO cells. Neuron. 1992 Apr;8(4):757–765. doi: 10.1016/0896-6273(92)90096-v. [DOI] [PubMed] [Google Scholar]
- Baird J. G., Challiss R. A., Nahorski S. R. Role for ionotropic and metabotropic receptors in quisqualate-stimulated inositol polyphosphate accumulation in rat cerebral cortex. Mol Pharmacol. 1991 Jun;39(6):745–753. [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and calcium signalling. Nature. 1993 Jan 28;361(6410):315–325. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and diacylglycerol: two interacting second messengers. Annu Rev Biochem. 1987;56:159–193. doi: 10.1146/annurev.bi.56.070187.001111. [DOI] [PubMed] [Google Scholar]
- Birrell G. J., Marcoux F. W. Excitatory amino acid receptor-stimulated phosphoinositide turnover in primary cerebrocortical cultures. Br J Pharmacol. 1993 Jun;109(2):379–385. doi: 10.1111/j.1476-5381.1993.tb13580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birse E. F., Eaton S. A., Jane D. E., Jones P. L., Porter R. H., Pook P. C., Sunter D. C., Udvarhelyi P. M., Wharton B., Roberts P. J. Phenylglycine derivatives as new pharmacological tools for investigating the role of metabotropic glutamate receptors in the central nervous system. Neuroscience. 1993 Feb;52(3):481–488. doi: 10.1016/0306-4522(93)90400-a. [DOI] [PubMed] [Google Scholar]
- Bleakman D., Rusin K. I., Chard P. S., Glaum S. R., Miller R. J. Metabotropic glutamate receptors potentiate ionotropic glutamate responses in the rat dorsal horn. Mol Pharmacol. 1992 Aug;42(2):192–196. [PubMed] [Google Scholar]
- Bockaert J., Pin J., Fagni L. Metabotropic glutamate receptors: an original family of G protein-coupled receptors. Fundam Clin Pharmacol. 1993;7(9):473–485. doi: 10.1111/j.1472-8206.1993.tb00252.x. [DOI] [PubMed] [Google Scholar]
- Boss V., Conn P. J. Metabotropic excitatory amino acid receptor activation stimulates phospholipase D in hippocampal slices. J Neurochem. 1992 Dec;59(6):2340–2343. doi: 10.1111/j.1471-4159.1992.tb10131.x. [DOI] [PubMed] [Google Scholar]
- Cerne R., Rusin K. I., Randić M. Enhancement of the N-methyl-D-aspartate response in spinal dorsal horn neurons by cAMP-dependent protein kinase. Neurosci Lett. 1993 Oct 29;161(2):124–128. doi: 10.1016/0304-3940(93)90275-p. [DOI] [PubMed] [Google Scholar]
- Challiss R. A., Mistry R., Gray D. W., Nahorski S. R. Modulatory effects of NMDA on phosphoinositide responses evoked by the metabotropic glutamate receptor agonist 1S,3R-ACPD in neonatal rat cerebral cortex. Br J Pharmacol. 1994 May;112(1):231–239. doi: 10.1111/j.1476-5381.1994.tb13057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins G. G. Actions of agonists of metabotropic glutamate receptors on synaptic transmission and transmitter release in the olfactory cortex. Br J Pharmacol. 1993 Feb;108(2):422–430. doi: 10.1111/j.1476-5381.1993.tb12820.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fain J. N., Berridge M. J. Relationship between phosphatidylinositol synthesis and recovery of 5-hydroxytryptamine-responsive Ca2+ flux in blowfly salivary glands. Biochem J. 1979 Jun 15;180(3):655–661. doi: 10.1042/bj1800655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison N. L., Simmonds M. A. Quantitative studies on some antagonists of N-methyl D-aspartate in slices of rat cerebral cortex. Br J Pharmacol. 1985 Feb;84(2):381–391. doi: 10.1111/j.1476-5381.1985.tb12922.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey J., Collingridge G. L. Signal transduction pathways involved in the acute potentiation of NMDA responses by 1S,3R-ACPD in rat hippocampal slices. Br J Pharmacol. 1993 Aug;109(4):1085–1090. doi: 10.1111/j.1476-5381.1993.tb13733.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irving A. J., Schofield J. G., Watkins J. C., Sunter D. C., Collingridge G. L. 1S,3R-ACPD stimulates and L-AP3 blocks Ca2+ mobilization in rat cerebellar neurons. Eur J Pharmacol. 1990 Sep 21;186(2-3):363–365. doi: 10.1016/0014-2999(90)90462-f. [DOI] [PubMed] [Google Scholar]
- Kelso S. R., Nelson T. E., Leonard J. P. Protein kinase C-mediated enhancement of NMDA currents by metabotropic glutamate receptors in Xenopus oocytes. J Physiol. 1992 Apr;449:705–718. doi: 10.1113/jphysiol.1992.sp019110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendall D. A., Nahorski S. R. Inositol phospholipid hydrolysis in rat cerebral cortical slices: II. Calcium requirement. J Neurochem. 1984 May;42(5):1388–1394. doi: 10.1111/j.1471-4159.1984.tb02799.x. [DOI] [PubMed] [Google Scholar]
- Kinney G. A., Slater N. T. Potentiation of NMDA receptor-mediated transmission in turtle cerebellar granule cells by activation of metabotropic glutamate receptors. J Neurophysiol. 1993 Feb;69(2):585–594. doi: 10.1152/jn.1993.69.2.585. [DOI] [PubMed] [Google Scholar]
- Manzoni O. J., Finiels-Marlier F., Sassetti I., Blockaert J., le Peuch C., Sladeczek F. A. The glutamate receptor of the Qp-type activates protein kinase C and is regulated by protein kinase C. Neurosci Lett. 1990 Feb 5;109(1-2):146–151. doi: 10.1016/0304-3940(90)90553-l. [DOI] [PubMed] [Google Scholar]
- Manzoni O. J., Poulat F., Do E., Sahuquet A., Sassetti I., Bockaert J., Sladeczek F. A. Pharmacological characterization of the quisqualate receptor coupled to phospholipase C (Qp) in striatal neurons. Eur J Pharmacol. 1991 Jul 12;207(3):231–241. doi: 10.1016/0922-4106(91)90035-g. [DOI] [PubMed] [Google Scholar]
- Markram H., Sakmann B. Calcium transients in dendrites of neocortical neurons evoked by single subthreshold excitatory postsynaptic potentials via low-voltage-activated calcium channels. Proc Natl Acad Sci U S A. 1994 May 24;91(11):5207–5211. doi: 10.1073/pnas.91.11.5207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markram H., Segal M. The inositol 1,4,5-trisphosphate pathway mediates cholinergic potentiation of rat hippocampal neuronal responses to NMDA. J Physiol. 1992 Feb;447:513–533. doi: 10.1113/jphysiol.1992.sp019015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller B., Sarantis M., Traynelis S. F., Attwell D. Potentiation of NMDA receptor currents by arachidonic acid. Nature. 1992 Feb 20;355(6362):722–725. doi: 10.1038/355722a0. [DOI] [PubMed] [Google Scholar]
- Minakami R., Katsuki F., Sugiyama H. A variant of metabotropic glutamate receptor subtype 5: an evolutionally conserved insertion with no termination codon. Biochem Biophys Res Commun. 1993 Jul 30;194(2):622–627. doi: 10.1006/bbrc.1993.1866. [DOI] [PubMed] [Google Scholar]
- Murphy S. N., Miller R. J. A glutamate receptor regulates Ca2+ mobilization in hippocampal neurons. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8737–8741. doi: 10.1073/pnas.85.22.8737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi S. Metabotropic glutamate receptors: synaptic transmission, modulation, and plasticity. Neuron. 1994 Nov;13(5):1031–1037. doi: 10.1016/0896-6273(94)90043-4. [DOI] [PubMed] [Google Scholar]
- Nedergaard S., Engberg I., Flatman J. A. The modulation of excitatory amino acid responses by serotonin in the cat neocortex in vitro. Cell Mol Neurobiol. 1987 Dec;7(4):367–379. doi: 10.1007/BF00733789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuman R. S., Ben-Ari Y., Gho M., Cherubini E. Blockade of excitatory synaptic transmission by 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) in the hippocampus in vitro. Neurosci Lett. 1988 Sep 23;92(1):64–68. doi: 10.1016/0304-3940(88)90743-4. [DOI] [PubMed] [Google Scholar]
- Niesen C., Charlton M. P., Carlen P. L. Postsynaptic and presynaptic effects of the calcium chelator BAPTA on synaptic transmission in rat hippocampal dentate granule neurons. Brain Res. 1991 Aug 2;555(2):319–325. doi: 10.1016/0006-8993(91)90358-3. [DOI] [PubMed] [Google Scholar]
- Palmer E., Monaghan D. T., Cotman C. W. Trans-ACPD, a selective agonist of the phosphoinositide-coupled excitatory amino acid receptor. Eur J Pharmacol. 1989 Aug 3;166(3):585–587. doi: 10.1016/0014-2999(89)90383-x. [DOI] [PubMed] [Google Scholar]
- Pin J. P., Duvoisin R. The metabotropic glutamate receptors: structure and functions. Neuropharmacology. 1995 Jan;34(1):1–26. doi: 10.1016/0028-3908(94)00129-g. [DOI] [PubMed] [Google Scholar]
- Pontzer N. J., Madamba S., Siggins G. R., Crews F. T. Concentrations of carbachol stimulating phosphoinositide hydrolysis cause a sustained decrease in membrane potential and firing rate: role of inositol and inositol polyphosphate second messengers. Brain Res. 1992 Dec 4;597(2):189–199. doi: 10.1016/0006-8993(92)91474-s. [DOI] [PubMed] [Google Scholar]
- Rahman S., McLean J. H., Darby-King A., Paterno G., Reynolds J. N., Neuman R. S. Loss of cortical serotonin2A signal transduction in senescent rats: reversal following inhibition of protein kinase C. Neuroscience. 1995 Jun;66(4):891–901. doi: 10.1016/0306-4522(95)00002-z. [DOI] [PubMed] [Google Scholar]
- Rahman S., Neuman R. S. Activation of 5-HT2 receptors facilitates depolarization of neocortical neurons by N-methyl-D-aspartate. Eur J Pharmacol. 1993 Feb 16;231(3):347–354. doi: 10.1016/0014-2999(93)90109-u. [DOI] [PubMed] [Google Scholar]
- Rahman S., Neuman R. S. Multiple mechanisms of serotonin 5-HT2 receptor desensitization. Eur J Pharmacol. 1993 Jul 20;238(2-3):173–180. doi: 10.1016/0014-2999(93)90845-9. [DOI] [PubMed] [Google Scholar]
- Rahman S., Neuman R. S. Myo-inositol reduces serotonin (5-HT2) receptor induced homologous and heterologous desensitization. Brain Res. 1993 Dec 24;631(2):349–351. doi: 10.1016/0006-8993(93)91557-9. [DOI] [PubMed] [Google Scholar]
- Reynolds J. N., Baskys A., Carlen P. L. The effects of serotonin on N-methyl-D-aspartate and synaptically evoked depolarizations in rat neocortical neurons. Brain Res. 1988 Jul 26;456(2):286–292. doi: 10.1016/0006-8993(88)90230-2. [DOI] [PubMed] [Google Scholar]
- Rhee S. G., Kim H., Suh P. G., Choi W. C. Multiple forms of phosphoinositide-specific phospholipase C and different modes of activation. Biochem Soc Trans. 1991 Apr;19(2):337–341. doi: 10.1042/bst0190337. [DOI] [PubMed] [Google Scholar]
- Rüegg U. T., Burgess G. M. Staurosporine, K-252 and UCN-01: potent but nonspecific inhibitors of protein kinases. Trends Pharmacol Sci. 1989 Jun;10(6):218–220. doi: 10.1016/0165-6147(89)90263-0. [DOI] [PubMed] [Google Scholar]
- Sayer R. J., Schwindt P. C., Crill W. E. Metabotropic glutamate receptor-mediated suppression of L-type calcium current in acutely isolated neocortical neurons. J Neurophysiol. 1992 Sep;68(3):833–842. doi: 10.1152/jn.1992.68.3.833. [DOI] [PubMed] [Google Scholar]
- Schoepp D. D., Conn P. J. Metabotropic glutamate receptors in brain function and pathology. Trends Pharmacol Sci. 1993 Jan;14(1):13–20. doi: 10.1016/0165-6147(93)90107-u. [DOI] [PubMed] [Google Scholar]
- Schoepp D. D., Johnson B. G., Smith E. C., McQuaid L. A. Stereoselectivity and mode of inhibition of phosphoinositide-coupled excitatory amino acid receptors by 2-amino-3-phosphonopropionic acid. Mol Pharmacol. 1990 Aug;38(2):222–228. [PubMed] [Google Scholar]
- Schoepp D., Bockaert J., Sladeczek F. Pharmacological and functional characteristics of metabotropic excitatory amino acid receptors. Trends Pharmacol Sci. 1990 Dec;11(12):508–515. doi: 10.1016/0165-6147(90)90052-a. [DOI] [PubMed] [Google Scholar]
- Sherman W. R., Gish B. G., Honchar M. P., Munsell L. Y. Effects of lithium on phosphoinositide metabolism in vivo. Fed Proc. 1986 Oct;45(11):2639–2646. [PubMed] [Google Scholar]
- Shigemoto R., Nakanishi S., Mizuno N. Distribution of the mRNA for a metabotropic glutamate receptor (mGluR1) in the central nervous system: an in situ hybridization study in adult and developing rat. J Comp Neurol. 1992 Aug 1;322(1):121–135. doi: 10.1002/cne.903220110. [DOI] [PubMed] [Google Scholar]
- Shigemoto R., Nomura S., Ohishi H., Sugihara H., Nakanishi S., Mizuno N. Immunohistochemical localization of a metabotropic glutamate receptor, mGluR5, in the rat brain. Neurosci Lett. 1993 Nov 26;163(1):53–57. doi: 10.1016/0304-3940(93)90227-c. [DOI] [PubMed] [Google Scholar]
- Tamaoki T., Nomoto H., Takahashi I., Kato Y., Morimoto M., Tomita F. Staurosporine, a potent inhibitor of phospholipid/Ca++dependent protein kinase. Biochem Biophys Res Commun. 1986 Mar 13;135(2):397–402. doi: 10.1016/0006-291x(86)90008-2. [DOI] [PubMed] [Google Scholar]
- Tanabe Y., Nomura A., Masu M., Shigemoto R., Mizuno N., Nakanishi S. Signal transduction, pharmacological properties, and expression patterns of two rat metabotropic glutamate receptors, mGluR3 and mGluR4. J Neurosci. 1993 Apr;13(4):1372–1378. doi: 10.1523/JNEUROSCI.13-04-01372.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thastrup O., Cullen P. J., Drøbak B. K., Hanley M. R., Dawson A. P. Thapsigargin, a tumor promoter, discharges intracellular Ca2+ stores by specific inhibition of the endoplasmic reticulum Ca2(+)-ATPase. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2466–2470. doi: 10.1073/pnas.87.7.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson A. M. Augmentation by glycine and blockade by 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) of responses to excitatory amino acids in slices of rat neocortex. Neuroscience. 1990;39(1):69–79. doi: 10.1016/0306-4522(90)90222-p. [DOI] [PubMed] [Google Scholar]
- Wojcikiewicz R. J., Tobin A. B., Nahorski S. R. Desensitization of cell signalling mediated by phosphoinositidase C. Trends Pharmacol Sci. 1993 Jul;14(7):279–285. doi: 10.1016/0165-6147(93)90131-3. [DOI] [PubMed] [Google Scholar]



