Abstract
1. We have investigated the actions of NS1619, a putative activator of large conductance calcium-activated potassium channels (BKCa) by use of the patch-clamp technique on smooth muscle cells enzymatically isolated from the rat basilar artery. 2. Using whole cell current-clamp to measure membrane potential, addition of 30 microM NS1619 produced cellular hyperpolarization, moving the membrane potential towards the calculated equilibrium potential for potassium. This hyperpolarization was rapidly reversed by IbTX (100 nM), a selective inhibitor of BKCa. 3. In whole cell recordings made from cells voltage-clamped at 0 mV using the perforated-patch technique, addition of NS1619 (10-30 microM) activated an outward current, which reversed following washout of NS1619. 4. This outward current was unaffected by application of either glibenclamide (5 microM), an inhibitor of ATP-sensitive potassium channels, or apamin (100 nM), an inhibitor of small-conductance calcium-activated potassium channels. However, this current was almost completely abolished by iberiotoxin (IbTX; 50-100nM). 5. Depolarizing voltage steps activated small outward currents from cells held at -15 mV. Application of NS1619 (10-30 microM) increased the size of these currents, producing a shift to the left of the current-voltage (I-V) relationship. These currents were largely inhibited by IbTX (100 nM). 6. Measurements of the unitary amplitude of the single channels activated by NS1619 which could be resolved in whole cell recordings yielded a value of 5.6 +/- 0.14 pA at 0 mV. 7. NS1619 (10-30 microM) directly activated single channels contained in excised inside-out and outside-out membrane patches. In both configurations NS1619 (10-30 microM) rapidly increased the open probability of a large conductance calcium-dependent channel. The activation produced by NS1619 was calcium-dependent and inhibited by external IbTX (100 nM). The unitary current amplitude was unaffected by NS1619. 8. By use of conventional whole cell recording methods and conditions that suppressed BKCa openings, outward potassium currents were activated by depolarizing potentials positive to -35 mV from a holding potential of -65 mV. NS1619 (10-30 microM) inhibited this current in a concentration-dependent manner. This inhibition was reversed following washout of NS1619, recovering to 60-90% of control values within 2 min. 9. Ba2+ currents, measured by conventional whole cell recording, were activated by depolarizing voltage steps from negative holding potentials. NS1619 (1-30 microM) inhibited the evoked current in a concentration-dependent manner, yielding an IC50 value of 7 microM with a Hill coefficient approaching unity. This inhibition was reversible, with the currents recovering to 65-100% of control values after washout of NS1619 for 2 min. 10. NS1619 (0.3-100 microM) induced concentration-dependent relaxation of basilar artery segments contracted with histamine/5-HT (IC50 = 12.5 +/- 2.0 microM; n = 4). This relaxation curve was shifted to the right, but not abolished, when the tissue was treated with a blocker of BKCa channels (IbTX; 100nM). Additionally, NS1619 produced concentration-dependent relaxation of basilar artery contracted with a depolarizing, isotonic salt solution containing 80 mM K+. 11. Thus NS1619 produces hyperpolarization of basilar artery myocytes through direct activation of BKCa and also directly inhibits Ca2+ currents and voltage-activated K+ channels. We discuss the implications of these results for its vasorelaxant actions.
Full text
PDF

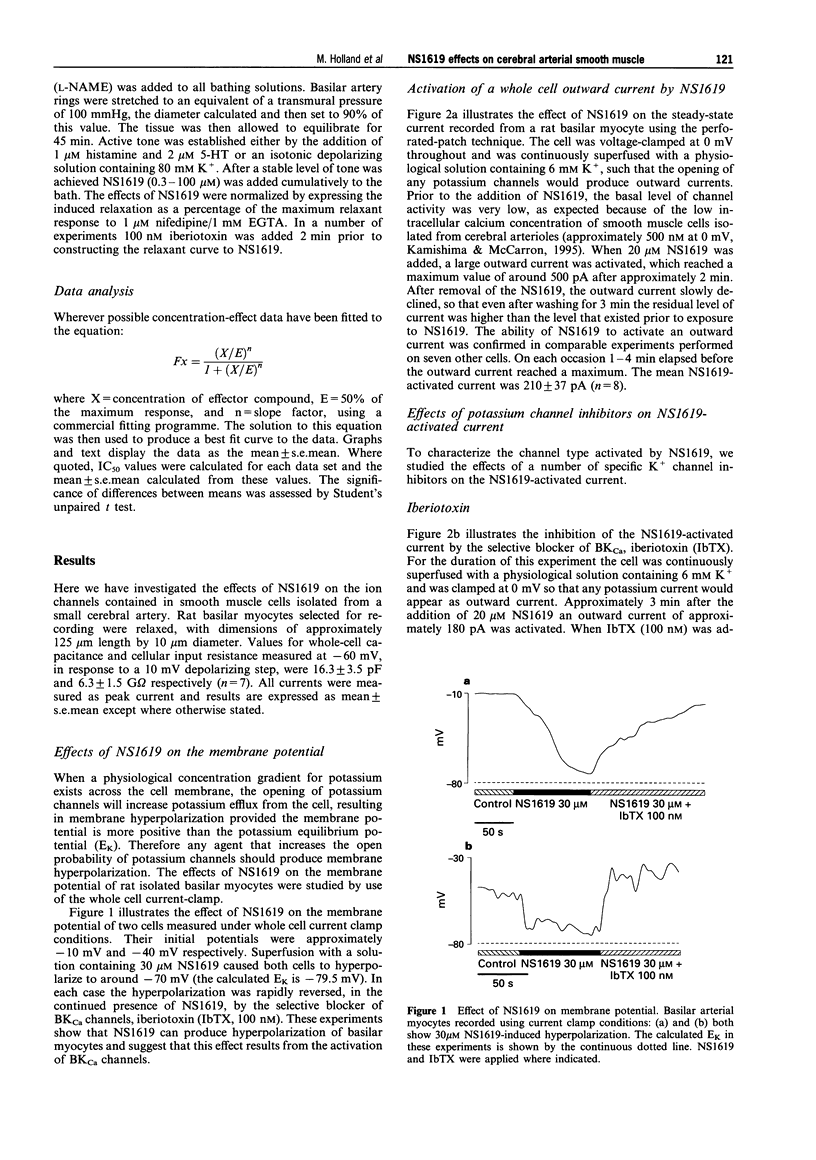
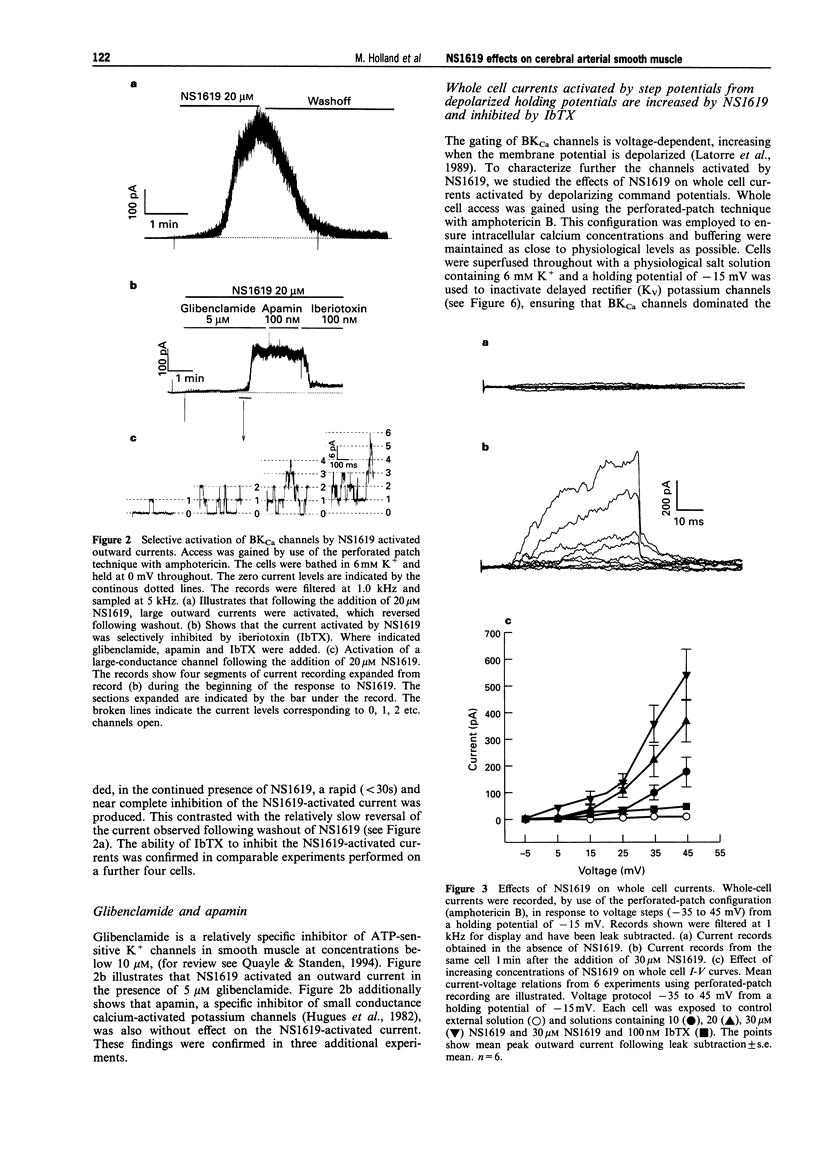


Selected References
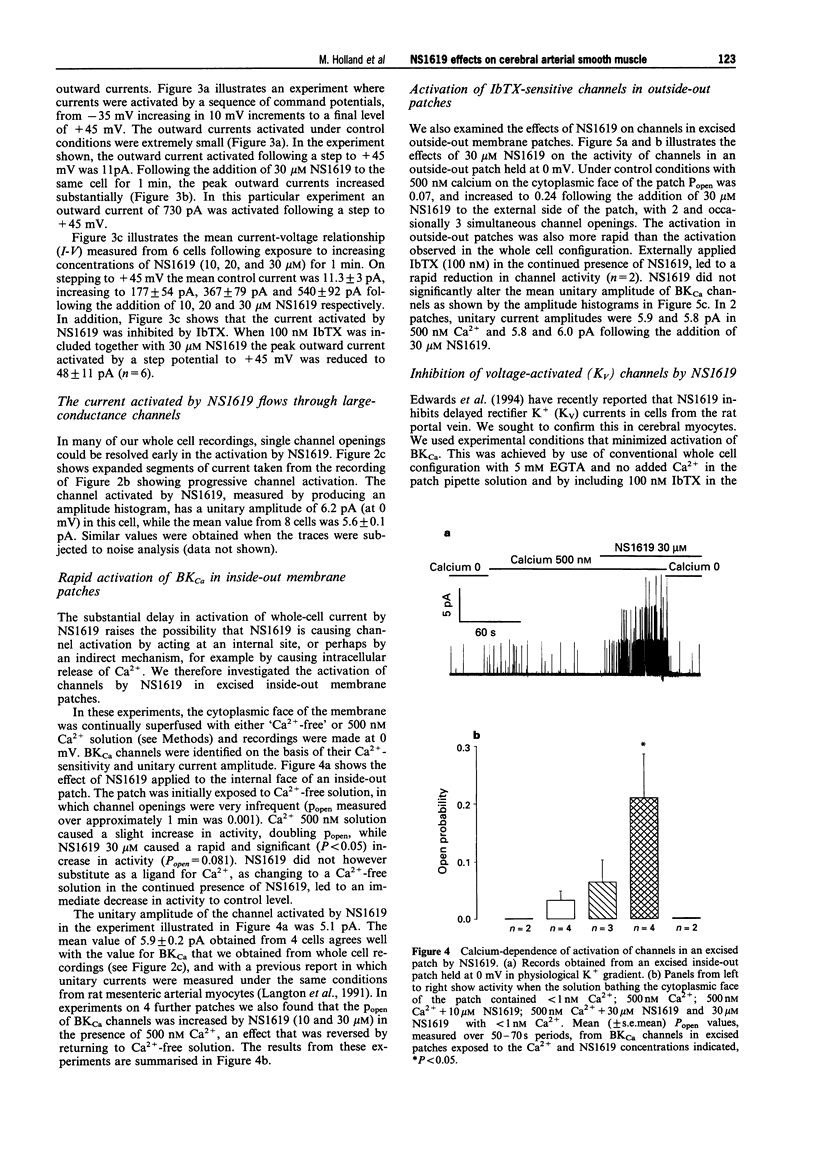
These references are in PubMed. This may not be the complete list of references from this article.
- Beech D. J., Bolton T. B. Two components of potassium current activated by depolarization of single smooth muscle cells from the rabbit portal vein. J Physiol. 1989 Nov;418:293–309. doi: 10.1113/jphysiol.1989.sp017841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brayden J. E., Nelson M. T. Regulation of arterial tone by activation of calcium-dependent potassium channels. Science. 1992 Apr 24;256(5056):532–535. doi: 10.1126/science.1373909. [DOI] [PubMed] [Google Scholar]
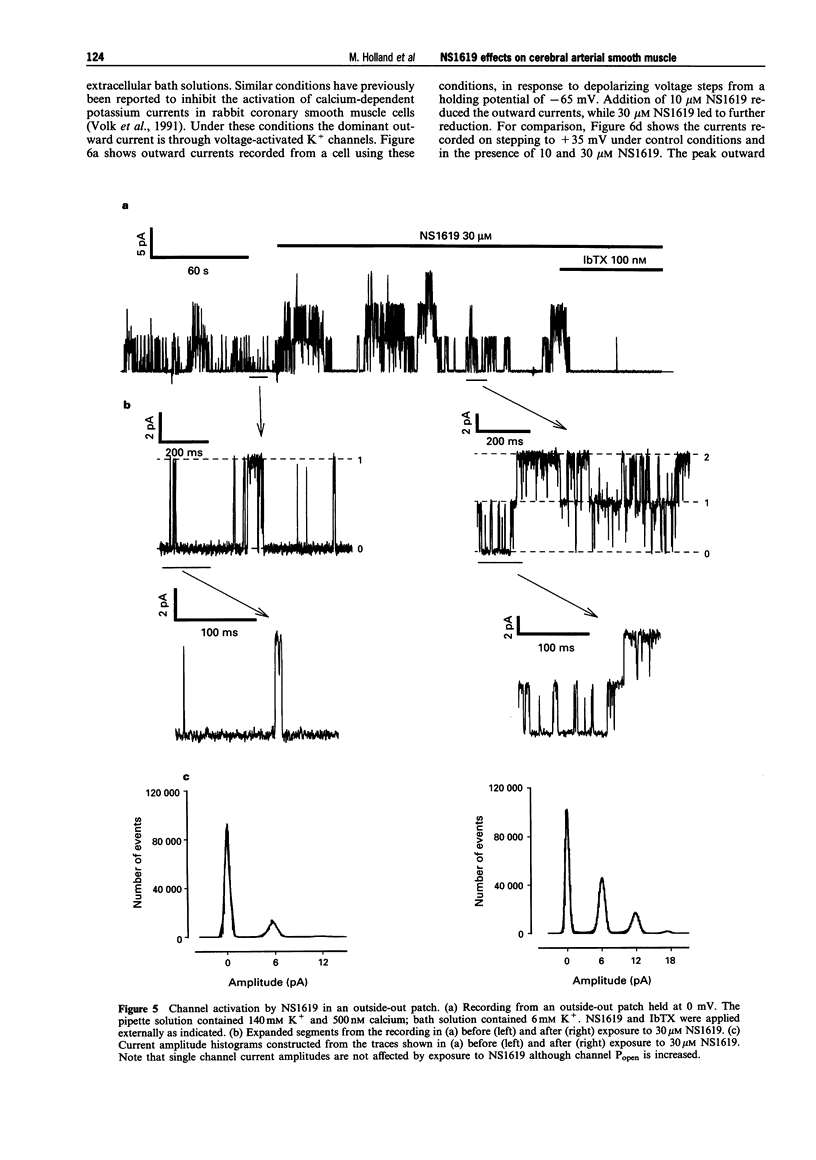
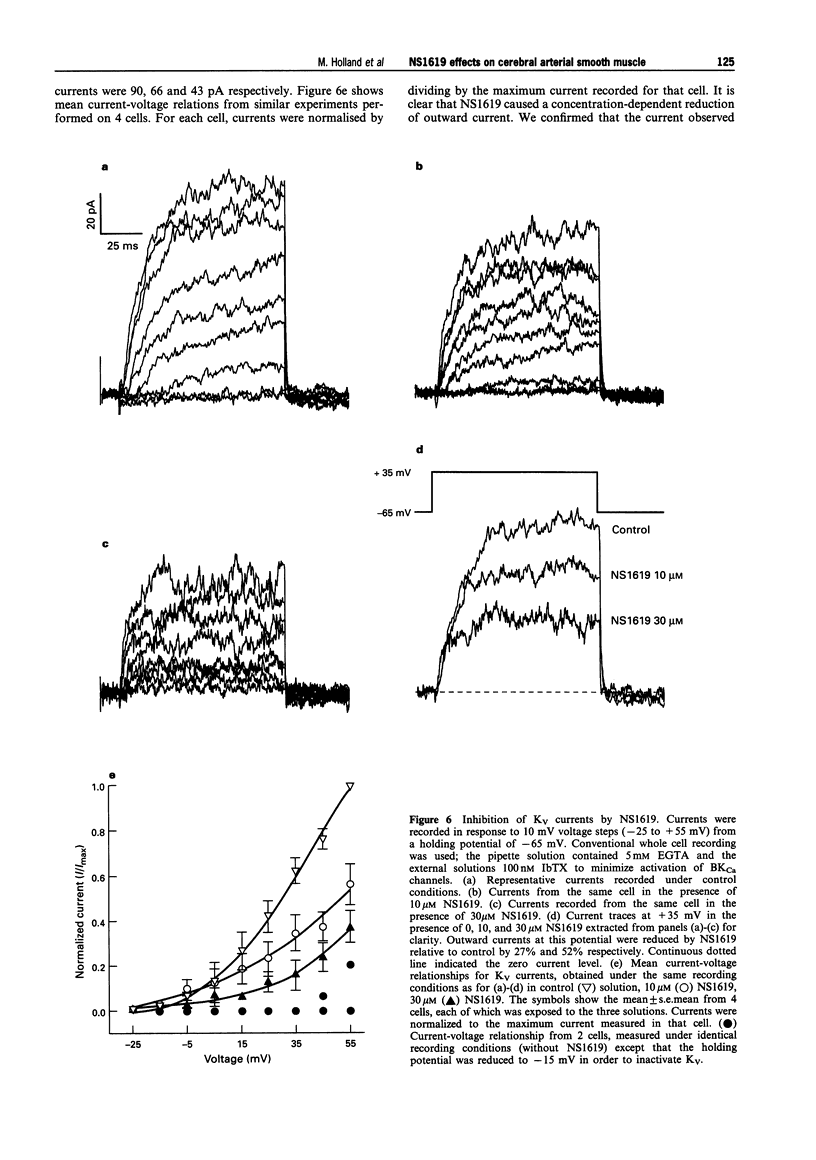
- Candia S., Garcia M. L., Latorre R. Mode of action of iberiotoxin, a potent blocker of the large conductance Ca(2+)-activated K+ channel. Biophys J. 1992 Aug;63(2):583–590. doi: 10.1016/S0006-3495(92)81630-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
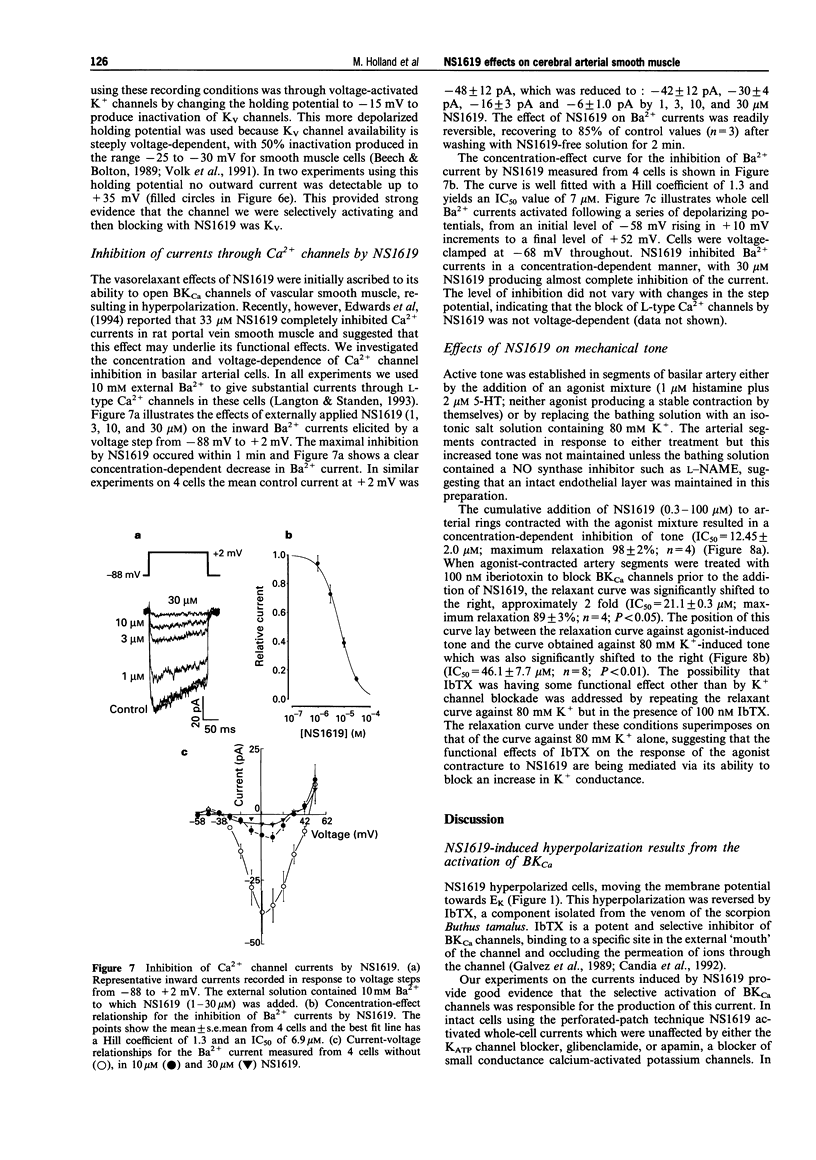
- Edwards G., Niederste-Hollenberg A., Schneider J., Noack T., Weston A. H. Ion channel modulation by NS 1619, the putative BKCa channel opener, in vascular smooth muscle. Br J Pharmacol. 1994 Dec;113(4):1538–1547. doi: 10.1111/j.1476-5381.1994.tb17171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
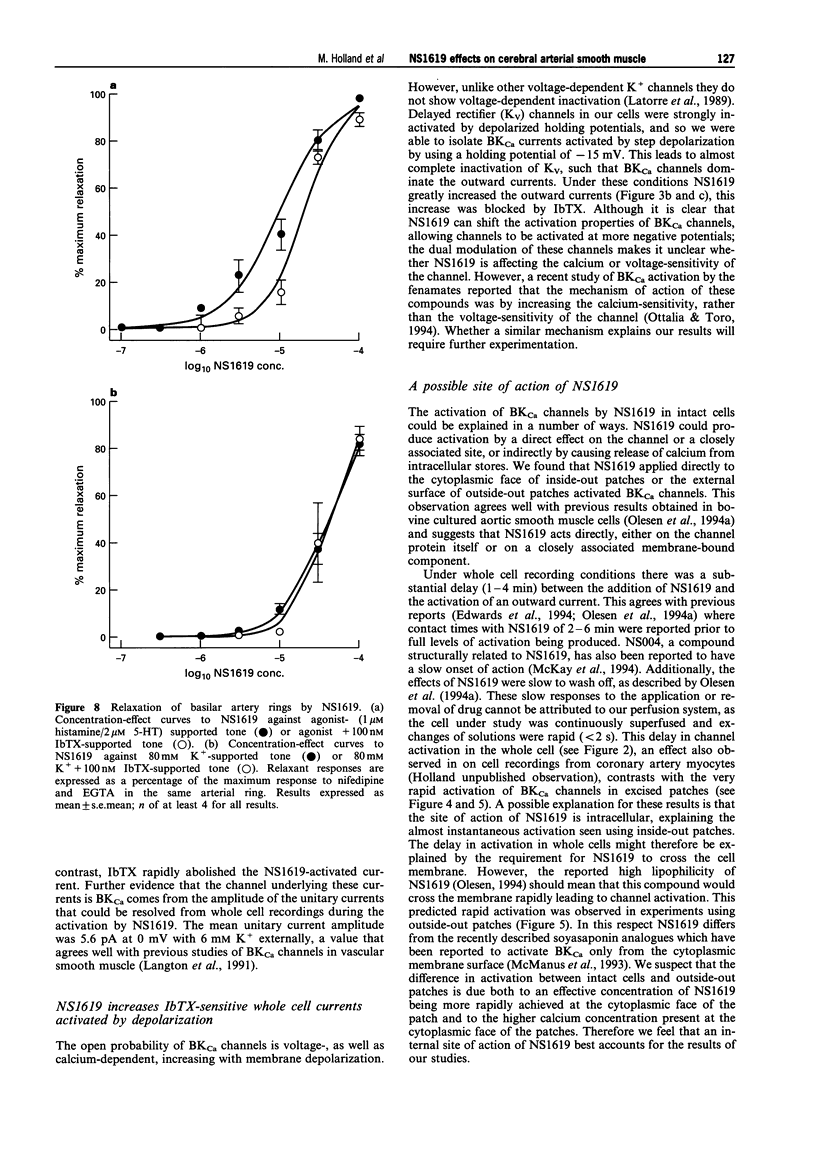
- Galvez A., Gimenez-Gallego G., Reuben J. P., Roy-Contancin L., Feigenbaum P., Kaczorowski G. J., Garcia M. L. Purification and characterization of a unique, potent, peptidyl probe for the high conductance calcium-activated potassium channel from venom of the scorpion Buthus tamulus. J Biol Chem. 1990 Jul 5;265(19):11083–11090. [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hugues M., Romey G., Duval D., Vincent J. P., Lazdunski M. Apamin as a selective blocker of the calcium-dependent potassium channel in neuroblastoma cells: voltage-clamp and biochemical characterization of the toxin receptor. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1308–1312. doi: 10.1073/pnas.79.4.1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langton P. D. Calcium channel currents recorded from isolated myocytes of rat basilar artery are stretch sensitive. J Physiol. 1993 Nov;471:1–11. doi: 10.1113/jphysiol.1993.sp019887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langton P. D., Nelson M. T., Huang Y., Standen N. B. Block of calcium-activated potassium channels in mammalian arterial myocytes by tetraethylammonium ions. Am J Physiol. 1991 Mar;260(3 Pt 2):H927–H934. doi: 10.1152/ajpheart.1991.260.3.H927. [DOI] [PubMed] [Google Scholar]
- Langton P. D., Standen N. B. Calcium currents elicited by voltage steps and steady voltages in myocytes isolated from the rat basilar artery. J Physiol. 1993 Sep;469:535–548. doi: 10.1113/jphysiol.1993.sp019828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latorre R., Oberhauser A., Labarca P., Alvarez O. Varieties of calcium-activated potassium channels. Annu Rev Physiol. 1989;51:385–399. doi: 10.1146/annurev.ph.51.030189.002125. [DOI] [PubMed] [Google Scholar]
- McKay M. C., Dworetzky S. I., Meanwell N. A., Olesen S. P., Reinhart P. H., Levitan I. B., Adelman J. P., Gribkoff V. K. Opening of large-conductance calcium-activated potassium channels by the substituted benzimidazolone NS004. J Neurophysiol. 1994 May;71(5):1873–1882. doi: 10.1152/jn.1994.71.5.1873. [DOI] [PubMed] [Google Scholar]
- Nelson M. T., Quayle J. M. Physiological roles and properties of potassium channels in arterial smooth muscle. Am J Physiol. 1995 Apr;268(4 Pt 1):C799–C822. doi: 10.1152/ajpcell.1995.268.4.C799. [DOI] [PubMed] [Google Scholar]
- Olesen S. P., Munch E., Moldt P., Drejer J. Selective activation of Ca(2+)-dependent K+ channels by novel benzimidazolone. Eur J Pharmacol. 1994 Jan 4;251(1):53–59. doi: 10.1016/0014-2999(94)90442-1. [DOI] [PubMed] [Google Scholar]
- Olesen S. P., Munch E., Wätjen F., Drejer J. NS 004--an activator of Ca(2+)-dependent K+ channels in cerebellar granule cells. Neuroreport. 1994 Apr 14;5(8):1001–1004. doi: 10.1097/00001756-199404000-00037. [DOI] [PubMed] [Google Scholar]
- Ottolia M., Toro L. Potentiation of large conductance KCa channels by niflumic, flufenamic, and mefenamic acids. Biophys J. 1994 Dec;67(6):2272–2279. doi: 10.1016/S0006-3495(94)80712-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quayle J. M., Bonev A. D., Brayden J. E., Nelson M. T. Calcitonin gene-related peptide activated ATP-sensitive K+ currents in rabbit arterial smooth muscle via protein kinase A. J Physiol. 1994 Feb 15;475(1):9–13. doi: 10.1113/jphysiol.1994.sp020045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quayle J. M., Standen N. B. KATP channels in vascular smooth muscle. Cardiovasc Res. 1994 Jun;28(6):797–804. doi: 10.1093/cvr/28.6.797. [DOI] [PubMed] [Google Scholar]
- Rae J., Cooper K., Gates P., Watsky M. Low access resistance perforated patch recordings using amphotericin B. J Neurosci Methods. 1991 Mar;37(1):15–26. doi: 10.1016/0165-0270(91)90017-t. [DOI] [PubMed] [Google Scholar]
- Sargent C. A., Grover G. J., Antonaccio M. J., McCullough J. R. The cardioprotective, vasorelaxant and electrophysiological profile of the large conductance calcium-activated potassium channel opener NS-004. J Pharmacol Exp Ther. 1993 Sep;266(3):1422–1429. [PubMed] [Google Scholar]
- Volk K. A., Matsuda J. J., Shibata E. F. A voltage-dependent potassium current in rabbit coronary artery smooth muscle cells. J Physiol. 1991 Aug;439:751–768. doi: 10.1113/jphysiol.1991.sp018691. [DOI] [PMC free article] [PubMed] [Google Scholar]



